
Evaluating the safety of setting positive end-expiratory pressure-guided transpulmonary pressure in acute respiratory distress syndrome: a prospective analysis
Highlight box
Key findings
• Optimizing and individualizing positive end-expiratory pressure (PEEP) is crucial for patient management.
• Adjusting PEEP settings based on positive transpulmonary end-expiratory pressure in acute respiratory distress syndrome (ARDS) patients does not adversely affect hemodynamic status.
• Complications related to esophageal pressure catheter placement were minimal, with 8.7% nosebleeds and 2.2% pneumothorax.
• The study found that the use of esophageal pressure catheters suggests the safety of the individualized PEEP approach.
What is known and what is new?
• PEEP optimization is critical in ARDS management but the safety of PEEP settings based on transpulmonary pressure is unclear.
• Our study showed that PEEP guided by transpulmonary end-expiratory pressure is safe for hemodynamic status for ARDS patients and has minimal complications with esophageal pressure catheter use.
What is the implication, and what should change now?
• PEEP adjustment-guided transpulmonary end-expiratory pressure could be safe for ARDS patients.
• Using esophageal pressure may be considered for optimizing PEEP settings in ARDS patients, particularly those with suspected increased pleural pressure or obesity.
Introduction
Acute respiratory distress syndrome (ARDS) is a multifactorial lung injury that is characterized by high rates of morbidity and mortality (1). High positive end-expiratory pressure (PEEP) is associated with improved mortality rates in patients with moderate to severe ARDS (2). In mechanical ventilation, it is essential to individualize and optimize PEEP levels for each ARDS patient, as increasing PEEP can increase the pressure on the right ventricle and cause hemodynamic impairment (3,4). No specific PEEP-setting approach has been proven to reduce mortality in moderate to severe ARDS patients, leaving no standard recommendation in current guidelines (1). Moreover, PEEP settings guided by transpulmonary pressure offer a strong physiological basis, showing improved oxygenation and respiratory mechanics over empirically set PEEP. Talmor et al. (5) introduced this concept in the EPVent trial, involving 61 patients, comparing PEEP guided by transpulmonary end-expiratory pressure (PL-exp) 0–10 cmH2O to the low PEEP/fraction of inspired oxygen (FiO2) approach. However, the safety impact of PEEP setting strategies guided by transpulmonary pressure remains insufficiently studied. Therefore, the objective of this study was to evaluate the safety and hemodynamic changes associated with PEEP guided by transpulmonary pressure and the complications related to the placement of esophageal pressure catheters. We present this article in accordance with the STROBE reporting checklist (https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-119/rc).
Methods
Study design
The study was a prospective study conducted in the mixed intensive care unit (ICU) of a tertiary hospital for patients with moderate to severe ARDS who were diagnosed according to the Berlin 2012 criteria (6) from November 2021 to October 2023.
The exclusion criteria included contraindications to the placement of esophageal pressure catheters, a history of chronic obstructive pulmonary disease, pneumothorax, severe coagulopathy, pulmonary embolism, lung transplantation, active bronchopleural fistula, neuromuscular disease, prone ventilation or extracorporeal membrane oxygenation, lack of adequate equipment, or a decline to participate in the study.
Protocols and measures
In the initial management of ARDS patients upon admission to the ICU (7), the PEEP setting is optimized based on the ARDSNetwork with the low-PEEP/FiO2 table protocols (8). Ventilation was initiated to optimize low tidal volumes ranging from 6 to 8 mL/kg of predicted body weight (PBW) and plateau pressures not above 30 cmH2O. When plateau pressures over 30 cmH2O at a tidal volume of 6 mL/kg PBW, a gradual reduction in tidal volume to 4 mL/kg PBW was acceptable, and establishing a new plateau pressure threshold of 35 cmH2O. Continuous monitoring ensured the optimization of oxygenation, targeting arterial oxygen saturation levels within the 88–95% range or maintaining the partial pressure of arterial oxygen (PaO2) between 55 and 80 mmHg. The respiratory rate was adjusted to remain below 35 breaths/minute, to achieve an arterial pH range of 7.30 to 7.45. Baseline values were established immediately post-optimization of the initial ventilatory settings guided by a low-PEEP/FiO2 table.
After the initial ventilation settings, an esophageal pressure balloon (Nutrivent™, Mirandola, Italy) was positioned in the patients. Determine the position of the balloon using a bedside chest X-ray guided by a radiopaque marker on the catheter balloon. Fully deflate the balloon to equalize pressure before the ventilator connection, then inflate incrementally from 0.5 to 8 mL. Dynamic occlusion tests were conducted to verify the correct positioning of the balloon and to measure the auto-PEEP at each data collection point. Dynamic occlusion test by compressing the chest during expiratory hold, monitoring the esophageal-to-airway pressure ratio. Correct catheter placement should show a 1:1 pressure change ratio, with acceptable deviations between 0.8 and 1.2 in the transpulmonary pressure waveform. Following this, PEEP adjustments were made using an esophageal balloon catheter, guided by transpulmonary end-expiratory pressure.
Patients were administered a continuous infusion of sedatives and analgesics following the Richmond Agitation-Sedation Scale score of −4 to −5, supplemented with rocuronium as required for neuromuscular blockers to control spontaneous breathing. Arterial blood gases were regularly assessed, and the intrinsic PEEP was measured before and after each PEEP adjustment to mitigate the risk of auto-PEEP, with careful adjustments made to the inspiratory/expiratory ratio. Subsequently, the PEEP was adjusted to ensure that the transpulmonary end-expiratory pressure remained above 0 and below 10 cmH2O, which was calculated using the following formula: transpulmonary end-expiratory pressure = total PEEP − esophageal end-expiratory pressure and transpulmonary end-inspiratory pressure = plateau pressure − esophageal end-inspiratory pressure. Maintaining a PL-exp above 0 in ARDS patients prevents alveolar collapse, reducing atelectrauma and improving oxygenation by keeping alveoli open and decreasing shunt from perfused but non-aerated areas, and keeping PL-exp below 10 cmH2O avoids excessive alveolar distention, reducing the risk of ventilator-induced lung injury.
We recorded changes in norepinephrine equivalents using the vasoactive-inotropic score (VIS) and tracked the cardiovascular Sequential Organ Failure Assessment (CV-SOFA) score before and after PEEP adjustments in 24 and 48 hours. The VIS was calculated as VIS = dopamine (µg/kg/min) + dobutamine (µg/kg/min) + 100 × epinephrine (µg/kg/min) + 100 × norepinephrine (µg/kg/min) + 10 × milrinone (µg/kg/min) + 10,000 × vasopressin (units/kg/min) + 50 × levosimendan (µg/kg/min).
Data collection
Patient monitoring included age, sex, and ARDS classification at ICU admission. All patients were assessed for arterial blood gas metrics, respiratory mechanics, and hemodynamic status. Additionally, the CV-SOFA score the VIS, and the Charlson Comorbidity Index (CCI) were evaluated at baseline and 24 and 48 hours post-admission. The primary outcome measures focused on the following hemodynamic parameters: systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, and VIS. Secondary outcomes included complication-related esophageal pressure catheter use and in-hospital mortality.
Statistical analysis
Statistical analyses were performed using R version 3.6.2. Continuous variables are presented as medians and interquartile ranges (IQR), and categorical variables are presented as frequencies and percentages. The data at baseline and after 24 and 48 hours were analyzed using repeated-measures analysis of variance (ANOVA), and overall temporal differences were assessed using the Friedman test. For significant changes in hemodynamic status and VIS, pairwise comparisons were conducted using ANOVA for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data, with P values adjusted via the Bonferroni correction. The significance threshold was set at P<0.05.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the Ethics Committee in Biomedical Research of Cho Ray Hospital (approval No. 1229/GCN-HDDD) on November 3, 2021. All participants in the study provided written informed consent, or their legally authorized representatives, were required to provide informed consent.
Results
The study conducted 116 ARDS patients admitted to the ICU from November 2021 to October 2023. Among these, 46 patients met our criteria for inclusion in the study, see Figure 1. The mean age was 49.8 years, with males accounting for 69.6% of our participants. Upon admission to the ICU, 76.1% of these patients were diagnosed with moderate ARDS. The median CV-SOFA score was 3 [IQR, 0–4], the VIS was 5.6 [IQR, 0–13.5], and the CCI score was 2 [IQR, 1–3]. However, none of the patients had a history of congestive heart failure, see Table 1. Our findings showed that 27 patients required increased PEEP to achieve a PL-exp >0 cmH2O, while none of the patients had a PL-exp above 10 cmH2O. The median increase in PEEP was 2 cm [IQR, 2–4 cm]. Blood pressure parameters, including DBP and SBP, showed no significant differences at various time points. However, the heart rate decreased from 114 to 106 beat per min over 48 hours, see Table 2. Moreover, severity scores including VIS, CV-SOFA score, and serum lactate, decreased significantly at 48 hours (P<0.05), as illustrated in Figure 2.

Table 1
| Characteristics | Data |
|---|---|
| Age (years) | 49.8±15.5 |
| Male | 32 (69.6) |
| BMI (kg/m2) | 24.7±4.4 |
| PBW (kg) | 58.5±6.5 |
| CV-SOFA score | 3 [0–4] |
| VIS | 5.6 [0–13.5] |
| CCI score | 2 [1–3] |
| ARDS classification | |
| Moderate (100< PaO2/FiO2 ≤200) | 35 (76.1) |
| Severe (PaO2/FiO2 ≤100) | 11 (23.9) |
| ARDS risk factors | |
| Pneumonia | 18 (39.1) |
| Sepsis or septic shock | 17 (37.0) |
| Lung contusion | 4 (8.7) |
| Pancreatitis | 4 (8.7) |
| Other | 3 (6.5) |
| Respiratory parameters | |
| Tidal volume (mL/kg PBW) | 6.7 [6.3–7.4] |
| PEEP (cmH2O) | 10.0 [8.0–10.0] |
| FiO2 (%) | 60 [51.5–80.0] |
| Respiratory rate (breaths/min) | 22.0 [20.0–25.0] |
Data are presented as mean ± SD, n (%), or median [IQR]. BMI, body mass index; PBW, predicted body weight; CV-SOFA, cardiovascular Sequential Organ Failure Assessment; VIS, vasoactive-inotropic score; CCI, Charlson Comorbidity Index; ARDS, acute respiratory distress syndrome; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; SD, standard deviation; IQR, interquartile range.
Table 2
| Parameters | Baseline | 24 hours | 48 hours | P value |
|---|---|---|---|---|
| Respiratory parameters | ||||
| PaO2/FiO2 | 135.8 [100.5–169.8] | 167.5 [140.8–232.7] | 203.6 [149.0–267.9] | <0.001†,‡ |
| Respiratory rate (rate/min) | 22.0 [20.0–25.0] | 22.0 [20.0–26.0] | 23.0 [20.0–26.0] | 0.77 |
| Plateau pressure (cmH2O) | 27.0 [24.0–29.0] | 26.0 [23.0–27.8] | 25.0 [22.0–27.0] | <0.001†,‡,§ |
| Respiratory system compliance (mL/cmH2O) | 23.8 [19.7–27.7] | 26.6 [24.6–30.8] | 26.7 [22.2–33.2] | < 0.001‡ |
| Transpulmonary end-expiratory pressure (cmH2O) | −0.8 [−1.4 to 0.78] | 1.2 [0.9–1.8] | 1.3 [0.7–1.7] | <0.001†,‡ |
| Esophageal end-expiratory pressure (cmH2O) | 9.7 [8.7–11.2] | 9.1 [8.7–10.8] | 8.7 [7.8–9.7] | <0.001†,‡ |
| Air blood gas | ||||
| pH | 7.4 [7.35–7.45] | 7.4 [7.34–7.44] | 7.4 [7.35–7.47] | 0.66 |
| PaCO2 (mmHg) | 39.9 [35.2–43.3] | 42.1 [36.4–44.3] | 39 [34.4–42.0] | 0.17 |
| PaO2 (mmHg) | 81.4 [67.2–97.4] | 81.4 [70.1–104.0] | 77 [65.6–93.2] | 0.47 |
| Hemodynamic parameters | ||||
| Heart rate (beat per min) | 114±19.6 | 107±20.6 | 106±18.7 | 0.01‡ |
| SBP (mmHg) | 122 [111–140] | 130 [120–140] | 128 [111–148] | 0.93 |
| DBP (mmHg) | 69 [60–70] | 64 [60–70] | 68 [60–74] | 0.37 |
| Lactate (mmol/L) | 2.2 [1.6–3.1] | 1.9 [1.3–2.6] | 1.8 [1.1–2.5] | <0.001†,‡,§ |
| VIS | 5.6 [0–13.5] | 0 [0–14.5] | 0 [0–4.4] | <0.001†,‡ |
| CV-SOFA | 3 [0–4] | 0 [0–4] | 0 [0–3] | <0.001†,‡ |
| Outcomes | ||||
| Ventilator days | 12 [8–20] | 12.0 [9.5–17.5] | 12.0 [8.0–22.0] | 0.73 |
| Length of hospital stay (days) | 19.0 [14.0–25.8] | 16.0 [14.0–21.0] | 21.0 [14.0–27.0] | 0.24 |
| In-hospital mortality | 18 (39.1) | 8 (42.1) | 10 (37.0) | 0.73 |
Data are presented as median [IQR], mean ± SD, or n (%). Variables with non-parametric distribution are presented using the median and IQR. The Friedman test was used for the statistical analysis, followed by post-hoc comparisons using the Wilcoxon test with Bonferroni adjustment for P values. †, a P value <0.05 for the comparison of baseline vs. 24 hours; ‡, a P value <0.05 for the comparison of baseline vs. 48 hours; §, a P value <0.05 for the comparison of 24 vs. 48 hours. PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; PaCO2, partial pressure of arterial carbon dioxide; SBP, systolic blood pressure; DBP, diastolic blood pressure; VIS, vasoactive-inotropic score; CV-SOFA, cardiovascular Sequential Organ Failure Assessment; IQR, interquartile range; SD, standard deviation.
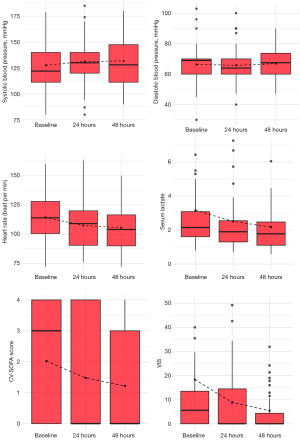
Complications related to esophageal pressure catheter placement were observed in a small number of patients: nosebleed or oral bleeding occurred in 4 patients (8.7%), gastric bleeding in 1 patient (2.2%), and pneumothorax in 2.2%. There were no cases of ventricular arrhythmia or esophageal rupture, as detailed in Table 3.
Table 3
| Complication-related esophageal pressure catheter | N (%) |
|---|---|
| Nosebleed/oral bleeding | 4 (8.7) |
| Gastric bleeding | 1 (2.2) |
| Esophageal rupture | 0 (0.0) |
| Catheter misplacement | 0 (0.0) |
| Ventricular arrhythmia | 0 (0.0) |
| Nausea | 0 (0.0) |
| New pneumothorax | 1 (2.2) |
| Pneumomediastinum | 0 (0.0) |
Discussion
ARDS is a multifactorial lung injury that is characterized by high rates of morbidity and mortality. Atelectrauma may increase from the regional forces generated during the cyclical closure and reopening of small airways (9,10). Negative PL-exp values are known to predispose to the closure of small airways, consequently leading to lung injury. This type of injury has been shown in preclinical models to be mitigated by the application of higher PEEP (11). The individualized adjustment of PEEP according to the PL-exp aligns with respiratory physiology. These strategies have been proven effective in improving blood oxygenation and respiratory mechanics (5,12). Furthermore, a recent observational study has shown the potential advantages of PL-exp measurements in specific patient subsets. Notably, a positive PL-exp was associated with a reduced 60-day mortality rate in obese patients (13). This finding emphasizes that, although the PL-exp generally correlates with a high-PEEP/FiO2 table at the population level, individual patient variations do exist. Esophageal pressure measurements can thus assist in tailoring PEEP management to each patient’s specific physiological needs. Recent recommendations also suggest the use of esophageal pressure in patients suspected of having increased pleural pressure or in obese individuals (14,15). However, in addition to these effects, excessively elevated settings of PEEP can precipitate alveolar overdistension, induce right ventricular dysfunction, and consequently lead to hemodynamic impairments (3).
To our knowledge, there are limited studies on the safety of esophageal pressure placement in ARDS patients. Sarge et al. (16) conducted a reanalysis of the VPVent trial involving 61 patients, comparing PEEP titration guided by esophageal pressure with a low PEEP/FiO2 group. The study found that the mean PEEP increase was 6 cmH2O, and hemodynamic parameters remained unchanged during the increase in PEEP. The mean arterial pressure (MAP) and CV-SOFA score were similar between the groups. The MAP slightly improved over the first 72 hours in both groups (P=0.576). The CV-SOFA score, fluid balance, urine output, and MAP also showed no differences at 72 hours (16). Wang et al. (17) reported comparing an esophageal pressure group with an ARDSNetwork group of 23 patients with traumatic ARDS. In addition to improvements in PaO2/FiO2 and lung compliance, there were no significant differences in DBP, SBP, or HR between the two groups (P>0.05). Furthermore, in arterial blood gas pH, partial pressure of arterial carbon dioxide (PaCO2), and lactate levels were not significantly different between these groups (P>0.05). The PEEP titration value in the esophageal pressure group was 12 cmH2O, which was significantly higher than the 8 cmH2O in the ARDSNetwork group. Our study showed similar results, DBP, SBP, and HR remained unchanged at 24 and 48 hours. The median SBP remained stable at 122 mmHg at baseline, 130 mmHg at 24 hours, and 128 mmHg at 48 hours. Similarly, Beitler et al. (12) found no significant hemodynamic compromise with individualized PEEP settings. Furthermore, in our study, lactate levels, the CV-SOFA, and VIS both significantly decreased over the 48 hours.
Additionally, complications related to the placement of esophageal pressure catheters were recorded in 4 patients with controllable nosebleeds and 1 patient with pneumothorax at 2.2%. Beitler et al. (12) reported that barotrauma occurred in 6 of 102 ARDS patients (5.9%), including 3 patients with pneumothorax (2.9%), pneumomediastinum, and subcutaneous emphysema. Of the 98 patients with PEEP set according to the high PEEP/FiO2 table, 5 patients had barotrauma including 2 pneumothorax patients (2.0%). In contrast, similar to our findings, Talmor et al. (5) reported no barotrauma events in 61 patients in EP-Vent trials managed with low PEEP/FiO2 settings and esophageal pressure guidance. Consequently, we cannot conclude a causal relationship between barotrauma and this situation.
Setting PEEP based on PL-exp did not result in a change in mortality compared to other studies. The LUNGSAFE trials showed that in-hospital mortality increases with the severity of ARDS, with in-hospital mortality rates of 34.9% [95% confidence interval (CI): 31.4–38.5%] for mild ARDS, 40.3% (95% CI: 37.4–43.3%) for moderate ARDS, and 46.1% (95% CI: 41.9–50.4%) for severe ARDS (18). The in-hospital mortality rate in our study was 39.1%. The median hospital stay was 19 days (IQR, 14–25.8 days). Similarly, Chen et al. (13) reported a 60-day mortality rate of 37.7%, and Beitler et al. (12) observed a 60-day mortality rate of 37.6% in patients in ventilator settings guided by PL-exp. In contrast, Talmor et al. (5) reported a lower mortality rate of only 17%, which including of patients with mild ARDS, leading to lower mortality.
These findings suggest that increasing PEEP as a component of a strategy to optimize transpulmonary pressure does not negatively impact hemodynamics, nor does it escalate the severity of illness by CV-SOFA scores or require an increase in vasopressor dose by VIS. In patients with normal lung function, elevated alveolar pressures without concurrent adequate volume expansion can compress the pulmonary vasculature, thereby elevating pulmonary vascular resistance (PVR), which may diminish cardiac output and impair right heart function (19,20). Conversely, in ARDS, which is characterized by reduced lung volumes and dominant atelectasis, PVR may also increase. Therefore, appropriate adjustments in PEEP could promote the recruitment of collapsed lung tissue, thereby reducing PVR. Thus, appropriately elevating PEEP to mitigate atelectasis and minimize overdistension could improve hemodynamic function. Furthermore, our study considered the safety of individualized PEEP adjustments, but the long-term impact on survival remains unclear. Further studies are needed to assess whether optimizing PEEP based on transpulmonary pressure can improve survival and long-term outcomes in ARDS patients, guiding future critical care practices.
Our study has several limitations. First, this was a single-center study with a small sample size, which may limit the generalizability of our findings. Second, we collected data on changes in hemodynamic management through vasopressor use only and were unable to monitor fluid balance, urine output, and kidney function markers, which may have been affected by PEEP adjustments and are critical for ARDS management. Third, we did not evaluate right ventricular function before and after PEEP adjustments, which would have provided a more comprehensive understanding of the impact of PEEP on right ventricular functions. Although sedation and paralytic agents were administered following standardized protocols to minimize variability, they remain potential confounders. However, the CV-SOFA and VIS scores showed no significant changes at 24 and 48 hours, it is unlikely that these agents had a major impact on hemodynamic stability in our study. Future studies should monitor these variables to confirm this observation.
Conclusions
Individualizing PEEP settings guided by positive transpulmonary end-expiratory pressure does not negatively affect hemodynamics. Esophageal pressure catheters are minimally invasive and safe. Further research is needed to more comprehensively evaluate hemodynamic status when adjusting PEEP based on transpulmonary pressure.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-119/rc
Data Sharing Statement: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-119/dss
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-119/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-119/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the Ethics Committee in Biomedical Research of Cho Ray Hospital (Approval No. 1229/GCN-HDDD) on November 3, 2021. All participants in the study provided written informed consent, or their legally authorized representatives, were required to provide informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grasselli G, Calfee CS, Camporota L, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 2023;49:727-59. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Fougères E, Teboul JL, Richard C, et al. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: importance of the volume status. Crit Care Med 2010;38:802-7. [Crossref] [PubMed]
- Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019;5:18. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Pintado MC, de Pablo R, Trascasa M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care 2013;58:1416-23. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Muscedere JG, Mullen JB, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994;149:1327-34. [Crossref] [PubMed]
- Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 2008;163:232-43. [Crossref] [PubMed]
- Loring SH, Pecchiari M, Della Valle P, et al. Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med 2010;38:2358-64. [Crossref] [PubMed]
- Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019;321:846-57. [Crossref] [PubMed]
- Chen L, Grieco DL, Beloncle F, et al. Partition of respiratory mechanics in patients with acute respiratory distress syndrome and association with outcome: a multicentre clinical study. Intensive Care Med 2022;48:888-98. [Crossref] [PubMed]
- Grotberg JC, Reynolds D, Kraft BD. Management of severe acute respiratory distress syndrome: a primer. Crit Care 2023;27:289. [Crossref] [PubMed]
- Baedorf Kassis E, Talmor D. Clinical application of esophageal manometry: how I do it. Crit Care 2021;25:6. [Crossref] [PubMed]
- Sarge T, Loring SH, Yitsak-Sade M, et al. Raising positive end-expiratory pressures in ARDS to achieve a positive transpulmonary pressure does not cause hemodynamic compromise. Intensive Care Med 2014;40:126-8. [Crossref] [PubMed]
- Wang B, Wu B, Ran YN. A clinical study on mechanical ventilation PEEP setting for traumatic ARDS patients guided by esophageal pressure. Technol Health Care 2019;27:37-47. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part II: practical implications in critical care. Intensive Care Med 2009;35:198-205. [Crossref] [PubMed]
- Spinelli E, Scaramuzzo G, Slobod D, et al. Understanding cardiopulmonary interactions through esophageal pressure monitoring. Front Physiol 2023;14:1221829. [Crossref] [PubMed]
Cite this article as: Nguyen TN, Trieu NHK, Pham TC, Pham TTN. Evaluating the safety of setting positive end-expiratory pressure-guided transpulmonary pressure in acute respiratory distress syndrome: a prospective analysis. J Emerg Crit Care Med 2025;9:10.


