
A rare complication in severe dengue: how point of care ultrasonography (POCUS) makes a difference?—a case report
Highlight box
Key findings
• Pulmonary embolism (PE) is identified as a rare complication of dengue viral infection.
• Point of care ultrasound (POCUS) can be used to differentiate various types of shock in severe dengue and help to guide an appropriate management.
What is known, and what is new?
• The most common complication of dengue virus infection is shock resulting from increased vascular permeability. This article reports an unusual case of obstructive shock due to PE, a rare complication of dengue viral infection. Managing this patient differs significantly from that of dengue shock syndrome and requires distinct interventions. Early identification of the underlying cause using POCUS is crucial for achieving favorable outcomes. However, invasive mechanical ventilation and lung hyperinflation, may impact the image quality obtained through POCUS.
• PE is a rare but treatable complication in patients with severe dengue infection when identified early. POCUS should be done routinely in patients with shock to delineate the cause of shock, which will be translated into different treatment strategies.
Introduction
Dengue fever is caused by flaviviruses that are transmitted by the female mosquitoes Aedes aegypti and Aedes albopictus. The dengue virus has four serotypes: DENV1, 2, 3, and 4. Infection with one serotype gives lifelong immunity against that serotype, but reinfection with another serotype increases the risk of developing severe dengue (1). Dengue fever has emerged as the most widespread and rapidly increasing vector-borne disease in the world. There are 3.5 billion people around the world living in dengue-endemic countries and at risk of contracting dengue fever. There are 1.3 billion people living in dengue-endemic areas in 10 countries of the Southeast Asia Region, including Indonesia (2). The incidence of dengue infection in Indonesia was reported at 5.07 per 100,000 people in 2022, with a case fatality rate of 0.86% (3).
Dengue infection has a wide spectrum of manifestations, ranging from asymptomatic infection to life-threatening severe dengue or dengue shock syndrome (DSS) (4). The World Health Organization (WHO) classifies dengue based on its level of severity into dengue with or without a warning sign and severe dengue. Severe dengue is characterized by severe plasma leakage and/or severe bleeding and/or severe organ impairment (5). In 2011, the WHO introduced the term “Expanded Dengue Syndrome” (EDS) to encompass a range of uncommon clinical manifestations that may affect neurological, hepatic, pulmonary, renal, and other isolated organs (6).
Severe forms of dengue are associated with systemic manifestations that involve almost every organ (7). The incidence of pulmonary manifestations is frequent and can be used as an indicator of the severity of disease. It can present as pleural effusion, pneumonitis, acute respiratory distress syndrome, or pulmonary hemorrhage (8). Recently, pulmonary embolism (PE) as a complication of dengue has been reported, although these reports are still limited to case reports (9,10). These complications are seen in more severe forms of dengue, which can have a complicated course and can be fatal if not managed properly and in a timely manner (7).
Point of care ultrasound (POCUS) is the acquisition, interpretation, and clinical integration of images performed by a treating clinician at the bedside. With this technique, the clinician can correlate real-time images with the patient’s presenting signs and symptoms (11,12). It can improve patient management by providing timely care, optimizing diagnostic accuracy, and improving procedural safety (13). Diagnostic POCUS complements history and physical examination to answer a specific clinical question, narrow differentials, guide clinical therapy, and direct consultations and disposition (14). In dengue, POCUS has been shown to be a useful adjunctive tool in the recognition, prognosis, management, and monitoring of patients (15-18). We present this case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-97/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s mother for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 9-year-old boy who had been treated for dengue hemorrhagic fever for 8 days in the pediatric ward was admitted to the pediatric intensive care unit (PICU) due to symptoms of respiratory failure and shock. He was unconscious, hypotensive, tachycardic, tachypneic, and cyanotic, with a SpO2 of 30% on PICU admission. Laboratory findings revealed a hemoglobin level of 8.9 g/dL, a leukocyte count of 10,800/µL, a platelet count of 73,000/µL, a positive C-reactive protein, a random blood sugar level of 193 mg/dL, elevated liver enzymes [aspartate aminotransferase (AST) of 2,151 U/L and alanine aminotransferase (ALT) of 1,724 U/L], normal renal function, normal electrolytes, and arterial blood gas that revealed mixed metabolic and respiratory acidosis (pH 7.049, PCO2 55.7 mmHg, pO2 163.5 mmHg, HCO3 14.7 meq/L). He was given 20 mL/kg body weight Ringer lactate; nonetheless, he continued to be hypotensive and showed progressive desaturation. He was eventually intubated and mechanically ventilated with a peak inspiratory pressure (PIP) of 26, a positive end-expiratory pressure (PEEP) of 14, an inspiratory-to-expiratory (I:E) ratio of 1:2, a respiratory rate of 25 breaths per minute, and a fraction of inspired oxygen (FiO2) of 100%. He required an epinephrine infusion to maintain blood pressure. The chest X-ray showed pulmonary edema with bilateral pleural effusion. A POCUS was performed with the RUSH protocol approach to evaluate the causes of shock and dyspnea. The pediatric-specific RUSH protocol is performed using the acronym HIPP [heart, inferior vena cava (IVC), pneumothorax, pelvis] (19). The results of the examination revealed an enlarged right atrium (RA) and right ventricle (RV) on the four-apical-chambers view, a D-shaped left ventricle and B lines on the PSAX view, hyperdynamic left ventricular (LV) function (Video 1), an IVC/Ao ratio of 1.9, bilateral pleural effusion, a distensibility inferior vena cava (dIVC) of 16.2%, phletoric IVC, pleural effusion and ascites. A continuous wave Doppler examination of the tricuspid valve was conducted to ascertain the tricuspid regurgitant jet velocity, which was subsequently employed to estimate the right ventricular systolic pressure (RVSP) using the simplified Bernoulli equation (20). From this measurement, the pressure gradient of the tricuspid valve was determined to be 37 mmHg. By estimating the right atrial pressure (RAP) as 10 mmHg, the RVSP can be calculated to be 47 mmHg (see Figure 1).
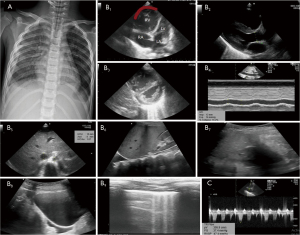
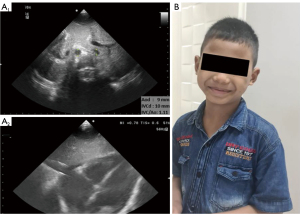
Further examination showed a D-dimer level of 10.0 mg/L (reference range, 0–0.5 mg/L), a prothrombin time (PT) of 35 s, and an activated partial thromboplastin time (APTT) of 39.8 s. Based on this data, it was concluded that he was diagnosed with obstructive shock due to PE. He was promptly treated with heparin and additional milrinone therapy. Ventilator settings, epinephrine and milrinone drip were gradually weaned. Two days later, POCUS monitoring in the subcostal view showed a normal appearance of all four chambers of the heart, and an inferior vena cava per aorta (IVC/Ao) ratio of 1.1 (Figure 2). He was successfully extubated on day 4, discharged from the PICU on day 6, and discharged from the hospital on day 9, with a prescription for oral aspirin for 3 months upon discharge.
Discussion
We report the role of POCUS in patient with dengue fever who develop shock and acute desaturation. One of the serious consequences of dengue virus infection is shock and low cardiac output. Shock in dengue generally occurs because of intravascular hypovolemia due to plasma leakage (10,21). The hemodynamics of dengue infection patients show that during plasma leakage, there is a decrease in stroke volume, cardiac index, left ventricle ejection fraction (LVEF), LV diastolic inflow, and an increase in vascular systemic resistance (22). The main principle of DSS management is to ensure adequate blood perfusion to vital organs with minimal fluid administration. Based on dengue management guidelines, the patient was treated with a fluid loading of 20 mL/kg body weight before admission to the PICU. However, this management did not improve the patient’s condition and further exacerbated acute desaturation. POCUS could not be performed due to the unavailability of an ultrasound device in our pediatric ward.
In patients with DSS who do not respond to adequate fluid therapy, it is essential to consider fluid overload or myocardial dysfunction as potential causes of hemodynamic compromise. In severe dengue, plasma leakage can occur rapidly across various organs due to increased vascular permeability during the defervescence phase. Uncontrolled fluid administration during this phase can lead to fluid overload with negative repercussions, such as respiratory distress and shock resulting from massive pleural effusion, pulmonary edema, and ascites due to circulatory overload (23-25). Fluid overload can also occur during fluid therapy in patients with comorbid conditions such as heart disease, chronic lung disease, and kidney disease (25). On the other hand, the underlying pathogenesis of myocardial dysfunction in dengue remains unclear but is thought to involve myocardial edema from local capillary leakage, the presence of circulating myocardial depressant factors (e.g., one or more proinflammatory mediators), coronary hypoperfusion, altered calcium homeostasis, or a combination of these factors (26). The risk of cardiac involvement in dengue virus infection is reported to be higher in the elderly population and lower in those under the age of 40 years old (27). A cross-sectional study of 130 children infected with the dengue virus over a 2-year period found an incidence of cardiac involvement due to dengue infection of 46.2%, with the majority of cases occurring in children with severe dengue (72.7%). Echocardiographic abnormalities identified included pericardial effusion (17.7%) and decreased LV ejection fraction (3.1%) (28). In this case, as the patient was unresponsive to fluid therapy, we considered the possibility of fluid overload or myocardial dysfunction. We promptly administered an epinephrine infusion considering myocardial dysfunction while performing POCUS to identify the etiology of the shock.
POCUS is a non-invasive and safe tool that is performed by bedside clinicians in early identifying the pathophysiological process of the disease, thereby accelerating the diagnostic process and improving the accuracy of diagnosis and management (17). Early diagnosis of shock in resource-limited countries is a challenge, especially with limited laboratory and radiology facilities. The development of POCUS has helped overcome these problems (29). Various multiorgan POCUS protocols have been reported for identifying the etiologic shock, and rapid ultrasound for shock and hypotension (RUSH) is the most comprehensive protocol and has good accuracy in differentiating the causes of shock (29,30). All proposed protocols are designed to answer specific questions about preload, contractility, and obstruction issues to help determine the etiology and adequate management of shock (19,20). Each protocol generally includes a standard heart view [parasternal long-axis (PLAX), parasternal short-axis (PSAX), apical four chamber, and subcostal four chamber view] for qualitative assessment of right and LV size and contraction as well as physiological assessment of pericardial fluid and tamponade. POCUS has a high sensitivity and positive likelihood ratio for determining the etiologic shock, especially in obstructive shock (30,31). The pediatric-RUSH protocol was derived from the adult RUSH protocol but did not evaluate abdominal aortic aneurysms. The pediatric-RUSH protocol consists of four distinct sonographic examinations, which can be easily remembered using the acronym HIPP (heart, IVC, pneumothorax, pelvis) (19). The use of POCUS in evaluating DSS patients with mechanical ventilation has been reported in Vietnam, and it was concluded that the use of POCUS was associated with a decreased risk of death, more fluid resuscitation, and improvements in serum lactate levels, vasoactive inotropic score, and PELOD-2 scores (17).
In this patient, a POCUS examination was performed to determine the cause of shock and dyspnea, and the results were an enlarged RV, RA, hyperdynamic left ventricle function, plethoric IVC, bilateral pleural effusion, and ascites. These findings definitively rule out myocardial dysfunction/cardiogenic shock and strongly support the diagnosis of obstructive shock. Obstructive shock is a condition caused by obstruction of major blood vessels or the heart itself (32). In dengue patients, obstructive shock is often found, especially in the advanced critical phase, which is generally caused by a drastic increase in intrathoracic pressure due to the accumulation of pericardial, pleural, and massive ascites and the use of mechanical ventilation (15). The severity of plasma leakage is associated with the size of pleural effusion and is inversely proportional to the early and late phases of LV wall movement during the diastolic phase (22). In this patient, the pleural effusion and ascites found were not significantly large, so we concluded that the cause of shock was not fluid accumulation.
Another cause of obstructive shock that has also been reported in patients with dengue virus infection, although rare, is PE. PE is a clinical condition caused by obstruction of the pulmonary artery and its branches by endogenous, exogenous emboli, or local thrombus formation (33). The incidence of thrombosis in dengue virus infection patients has so far only been reported as case reports (4,10,33-35). A report from Brazil reported a thrombotic incidence of 5.4% in hospitalized dengue patients, with diagnoses of deep vein thrombosis, pulmonary, and mesenteric vein thrombosis (34). Until now, the pathophysiology of thrombosis in dengue infection is not clear, but it is thought to be related to the inflammatory condition caused by dengue virus infection (10). The incidence of thrombosis is thought to be higher if the infection is prolonged. Most thrombotic events occur after 7 days of fever onset, especially when the patient is no longer in the acute phase (4). This is like our case, which occurred after 7 days of fever.
The pathophysiology of PE begins with pulmonary artery obstruction, which will increase vascular load (afterload) on the RV (36). The sudden increase in RV afterload will cause an increase in RV wall tension with a consequent decrease in right coronary artery flow, increased myocardial oxygen consumption and demand, and ischaemia (37). In the compensation stage, the RV contractile reserve will adapt to the increased afterload by increasing RV contractility in response to the increased afterload, ensuring that ventriculo-arterial (VA) coupling is maintained when there is an increase in metabolic demand (1). To maintain cardiac output, the RV will dilate and cause increased shear stress on the RV wall with consequent leftward septal bowing (36,38,39). Severe RV dilation will also stretch the tricuspid annulus and cause tricuspid regurgitation (40). In the decompensation stage, the RV contractile reserve can no longer adapt to the increase in afterload (the increase in afterload is not accompanied by an increase in RV contraction anymore), resulting in right ventricular-pulmonary artery (RV-PA) uncoupling, which further leads to RV dysfunction and right heart failure (36,38,41). The diagnosis of PE needs to be fast, but this is quite challenging considering that the clinical manifestations are generally non-specific and often misinterpreted with other medical emergencies (42). Most patients show respiratory symptoms such as dyspnea or chest pain, palpitations, and cough. Physical examination findings are generally tachypnea, tachycardia, decreased breath sounds, cyanosis, and swelling of the extremities (33,40). The chest X-ray is generally non-specific and can be used to exclude pneumonia or pneumothorax. Echocardiography is recommended, especially in patients with suspected PE with unstable hemodynamics, if an emergency computed tomography pulmonary angiogram (CTPA) is not available. The gold standard for diagnosing PE in children is the CTPA (43), but unfortunately it is not available in our hospital.
In this case, the chest X-ray showed pulmonary edema. Although acute pulmonary edema is often attributed to LV failure, it must kept in mind that, acute pulmonary edema may not be due to LV failure (44). Acute pulmonary edema has been reported in cases of PE. The association of acute pulmonary edema as a complication of PE is well known (45-47). However, the relationship between these two conditions is not widely understood by clinicians, and the underlying diagnosis of PE in patients with pulmonary edema may be missed (45). The mechanism of pulmonary edema in cases of pulmonary edema can be due to overperfusion in non-thrombosed areas resulting in increased hydrostatic pressure and flow in these areas (45,46) or due to the release of inflammatory mediators in hypoperfused areas (47). The implications of pulmonary edema in PE are (I) decreased gas exchange efficiency in the emboli lung, (II) concentration of blood flow to the healthy lung resulting in pulmonary edema and further reducing gas exchange efficiency and increasing hydrostatic pressure, (III) decreased gas exchange and obstructive shock will cause acidemia and trigger progressive circulatory failure (46).
The echocardiographic features that support the diagnosis of acute PE are RV pressure overload and dysfunction [right ventricular (RV) strain] (43,48). Echocardiography findings of PE in children can be divided into: (I) qualitative findings such as the presence of thrombus in the RA, RV, or branch of the pulmonary artery; flattened interventricular septum/D-shaped LV; paradoxical septal motion; McConnell sign: hypokinesia of the RV free wall; and qualitatively depressed RV function; (II) quantitative findings such as evidence of RV dilatation through a RV/LV ratio >0.66 and tricuspid valve regurgitation with increased RV pressure and decreased tricuspid annular plane systolic excursion (TAPSE) (40,48). The D-shaped appearance and McConnell’s sign have high specificity for PE at 96.2% and 98.6%, respectively. However, normal echocardiographic findings cannot exclude PE, considering its low effectiveness in distal embolism and its greater effectiveness in central embolism (33,43).
Echocardiography can estimate RVSP by measuring the velocity of tricuspid valve regurgitation using the modified Bernoulli equation. The velocity of the tricuspid regurgitation jet indicates the pressure gradient between the RV and the RA. Consensus guidelines recommend further evaluation if RVSP exceeds 40 mmHg or if tricuspid regurgitation velocity (TRV) is greater than 2.8 m/s, particularly in cases of unexplained dyspnea or RV dysfunction (49). Assessment of right heart systolic function can be performed through the measurement of TAPSE. TAPSE provides a longitudinal assessment of RV systolic function by measuring the movement of the tricuspid annulus toward the apex. This measurement is obtained using M-mode from the apical four-chamber view, with the cursor placed on the lateral tricuspid annulus (49,50). This measurement is easier than RV ejection fraction measurement in clinical practice. Normal reference values for TAPSE in the pediatric population have been published as percentile charts for the evaluation of RV systolic function (51).
In our patient, the POCUS evaluation using the RUSH protocol demonstrated: enlarged RV, D-shaped left ventricle, McConnell’s sign on the RV, hyperdynamic left ventricle function, plethoric IVC, IVC/Ao ratio of 1.9, dIVC 16.2%, bilateral pleural effusions, mild ascites (Morrison’s pouch, splenorenal recess, and urinary bladder), and no signs of cardiac tamponade or pneumothorax. In this patient, the assessment of fluid responsiveness was performed using the IVC/aorta ratio rather than the dIVC. The diameter of the IVC is influenced by respiratory changes and fluid deficiency, while the aorta remains relatively stable even in dehydration due to its lower compliance compared to the IVC, making the aorta a useful internal control. However, it is important to note that longitudinal M-mode measurement of the IVC can occasionally yield inaccurate readings due to factors such as probe positioning (centered or edge of the IVC) or the angle of the probe relative to the IVC (49).
Unfortunately, during the follow-up evaluation, we were unable to obtain apical four-chamber, PLAX, and PSAX views due to the patient being supported with high-pressure mechanical ventilation. Consequently, we could only assess the heart through a subcostal view. The interpretation of POCUS requires the acquisition of an adequate transthoracic image. Invasive mechanical ventilation and pulmonary hyperinflation often compromise visibility, as the cardiac window narrows and lung parenchyma interposes between the transducer and the heart, leading to reverberation artifacts from pleural lines and lung parenchyma. Patients undergoing mechanical ventilation may find it challenging to obtain parasternal or apical views because PEEP can shift the heart inferiorly due to diaphragm flattening. Nevertheless, qualitative assessment of RV and LV function and size, as well as the presence of pericardial effusion, demonstrates strong concordance between subcostal and parasternal/apical views (49).
A D-dimer examination was performed, and a high level of 10 mg/L was obtained. A multicenter study in Turkey on PE patients reported that all PE patients had increased serum D-dimer levels. Findings of high D-dimer levels accompanied by pulmonary symptoms in adults indicate that the patient is likely to have PE, but this condition does not apply in the pediatric population (33). Low D-dimer levels can be used to exclude PE in children (33,50). Furthermore, elevated D-dimer levels have also been observed in adult (51) and pediatric patients (50,52) infected with dengue. The clinical manifestation and POCUS findings were the basis for establishing high risk PE and considering heparinization in our patient. Haemodynamic support, including the administration of epinephrine and milrinone, was also provided to our patient. The use of heparinisation and haemodynamic support in our patient likely accelerated the healing process, resulting in a favourable outcome. The patient was discharged on oral aspirin for a period of 3 months.
In patients with high-risk and intermediate PE, immediate anticoagulation is recommended (40,53). For secondary prevention, oral anticoagulant therapy may be administered for a period of 3 months (54). The recommended supportive therapy for hemodynamic support in children is epinephrine (40). Fluid resuscitation should only be given if there is hypovolemia and absent RV dilatation. The use of inodilators (levosimendan, milrinone, and amrinone) has been demonstrated to alleviate increased PA pressure and RV afterload. This is achieved by counteracting vasoconstrictive processes and by increasing the non-obstructed cross-sectional area for blood flow. However, their use should be cautious as they may cause peripheral vasodilatation, thereby reducing right coronary perfusion and potentially worsening RV function (54).
Conclusions
It is necessary to consider other causes of shock in patients with severe dengue besides increased vascular permeability, namely PE, especially in patients with unstable hemodynamics associated with acute desaturation. Early evaluation of a DSS patient with POCUS can help establish various types of shock, which will then affect the management and outcome of the patient. However, although POCUS assists in diagnosis and therapy monitoring, it cannot replace thorough anamnesis, physical examination, clinical judgment, and formal imaging modalities.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-97/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-97/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-97/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s mother for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tayal A, Kabra SK, Lodha R. Management of Dengue: An Updated Review. Indian J Pediatr 2023;90:168-77. [Crossref] [PubMed]
- WHO. Available online: https://www.who.int/southeastasia/health-topics/dengue-and-severe-dengue. Dengue in the South-East Asia.
- Samad I, Handito A, Sugiarto A, et al. Laporan Tahunan 2022 Demam Berdarah Dengue. Jakarta: Unicef Indonesia; 2023:15-9.
- Nugraha ID. The Incidence of Venous Thromboembolism in Dengue Viral Infections: A Systematic Review. ASEAN Heart Journal 2022;31:13-6. [Crossref]
- Dengue guidelines for diagnosis, treatment, prevention and control treatment, prevention and control treatment, prevention and control [Internet]. Available online: www.who.int/tdr
- World Health Organization. Regional Office for South-East Asia. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. World Health Organization Regional Office for South-East Asia; 2011:196.
- Fotedar S, Singh J, Garg A, et al. Pulmonary Manifestations of Dengue Fever Section at a Tertiary Care Centre in Northern India: A Cross-sectional Study. Journal of Clinical and Diagnostic Research 2023;17:OC14-OC18. [Crossref]
- Gupta S, Singh L, Tandon R. Study of Pulmonary Manifestations among Dengue Patients in Tertiary Care Hospital of North India. International Journal of Contemporary Medical Research 2020;7: [Crossref]
- Kamath SD, Kumar M, Sunder A. Pulmonary Thromboembolism Complicating Dengue Fever—The Other Side of Dengue! Infectious Diseases in Clinical Practice 2021;29:e239-41. [Crossref]
- Poletto F, Cerruti L, Spiezia L. Dengue fever as a rare cause of pulmonary embolism. J Thromb Thrombolysis 2020;49:690-3. [Crossref] [PubMed]
- Burton L, Bhargava V, Kong M. Point-of-Care Ultrasound in the Pediatric Intensive Care Unit. Front Pediatr 2021;9:830160. [Crossref] [PubMed]
- Le Coz J, Orlandini S, Titomanlio L, et al. Point of care ultrasonography in the pediatric emergency department. Ital J Pediatr 2018;44:87. [Crossref] [PubMed]
- Ali N, Shakeel E, Soomar SM. Need of Point of Care Ultrasound Training in Pediatric Emergency Medicine Practice: A Wake-Up Call for the Low-Income Countries. Glob Pediatr Health 2023;10:2333794X231187485.
- Conlon TW, Nishisaki A, Singh Y, et al. Moving Beyond the Stethoscope: Diagnostic Point-of-Care Ultrasound in Pediatric Practice. Pediatrics 2019;144:e20191402. [Crossref] [PubMed]
- Nguyen TT, Le NT, Nguyen NM, et al. Clinical features and management of children with dengue-associated obstructive shock syndrome: A case report. Medicine (Baltimore) 2022;101:e31322. [Crossref] [PubMed]
- Osorio L, Prieto I, Zuluaga D, et al. Evaluation of remote radiologist-interpreted point-of-care ultrasound for suspected dengue patients in a primary health care facility in Colombia. Infect Dis Poverty 2023;12:90. [Crossref] [PubMed]
- Vo LT, Nguyen DT, Tran TN, et al. Pediatric Profound Dengue Shock Syndrome and Use of Point-of-Care Ultrasound During Mechanical Ventilation to Guide Treatment: Single-Center Retrospective Study, 2013-2021. Pediatr Crit Care Med 2024;25:e177-85. [Crossref] [PubMed]
- Gleeson T, Pagnarith Y, Habsreng E, et al. Dengue Management in Triage using Ultrasound in children from Cambodia: a prospective cohort study. Lancet Reg Health West Pac 2022;19:100371. [Crossref] [PubMed]
- Weberding NT, Marin JR. Point-of-Care Ultrasound for Targeted Assessment of Shock Remember HIPP in Children [Internet]. 2019. Available online: www.pec-online.com
- Binder ZW, O'Brien SE, Boyle TP, et al. Tricuspid Regurgitant Jet Velocity Point-of-Care Ultrasound Curriculum Development and Validation. POCUS J 2021;6:88-92. [Crossref] [PubMed]
- Rusmawatiningtyas D, Aditya Wiguna P, Fatah Kumara I, et al. Initial Hemodynamic Profiles of Children with Dengue Shock Syndrome in Referral Settings. American Journal of Pediatrics 2019;5:260-6. [Crossref]
- Kirawittaya T, Yoon IK, Wichit S, et al. Evaluation of Cardiac Involvement in Children with Dengue by Serial Echocardiographic Studies. PLoS Negl Trop Dis 2015;9:e0003943. [Crossref] [PubMed]
- Diana BN, Yenny CB, Maria Carolina MB, et al. Fluid Management in Dengue Critical Phase: Which, When, How Much? Int Arch Med Microbiol 2022. DOI:
10.23937/2643-4008/1710015 .10.23937/2643-4008/1710015 - Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog 2016;12:e1005738. [Crossref] [PubMed]
- Soegijanto S, Chilvia E. Update management dengue shock syndrome in pediatric cases. Indonesian Journal of Tropical and Infectious Disease 2013;4:9-22. Available online:
10.20473/ijtid.v4i4.227 10.20473/ijtid.v4i4.227 - Yacoub S, Wertheim H, Simmons CP, et al. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol 2014;11:335-45. [Crossref] [PubMed]
- Wei KC, Wang WH, Wu CL, et al. Heart failure after dengue infection- a population-based self-controlled case-series study. Travel Med Infect Dis 2023;53:102589. [Crossref] [PubMed]
- Abhinayaa J, James S, Jebaraj R, et al. Incidence of Cardiac Manifestations in Children with Dengue Fever: A Cross-sectional Study. Rambam Maimonides Med J 2021;12:e0014. [Crossref] [PubMed]
- Rahulkumar HH, Bhavin PR, Shreyas KP, et al. Utility of Point-of-Care Ultrasound in Differentiating Causes of Shock in Resource-Limited Setup. J Emerg Trauma Shock 2019;12:10-7. [Crossref] [PubMed]
- Stickles SP, Carpenter CR, Gekle R, et al. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. CJEM 2019;21:406-17. [Crossref] [PubMed]
- Yoshida T, Yoshida T, Noma H, et al. Diagnostic accuracy of point-of-care ultrasound for shock: a systematic review and meta-analysis. Crit Care 2023;27:200. [Crossref] [PubMed]
- Standl T, Annecke T, Cascorbi I, et al. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch Arztebl Int 2018;115:757-68. [PubMed]
- Hangül M, Köse M, Pekcan S, et al. Pulmonary Embolism in Childhood: A Multicenter Experience from Turkey. Balkan Med J 2022;39:366-73. [Crossref] [PubMed]
- da Costa PS, Ribeiro GM, Junior CS, et al. Severe thrombotic events associated with dengue fever, Brazil. Am J Trop Med Hyg 2012;87:741-2. [Crossref] [PubMed]
- Ranasinghe KMIU, Dissanayaka D, Thirumavalavan K, et al. An unusual case of dengue shock syndrome complicated by ilio-femoral deep vein thrombosis; a case report. BMC Infect Dis 2020;20:335. [Crossref] [PubMed]
- Grünig E, Eichstaedt CA, Seeger R, et al. Right Heart Size and Right Ventricular Reserve in Pulmonary Hypertension: Impact on Management and Prognosis. Diagnostics (Basel) 2020;10:1110. [Crossref] [PubMed]
- Edward J, Banchs J, Parker H, et al. Right ventricular function across the spectrum of health and disease. Heart 2023;109:349-55. [PubMed]
- He Q, Lin Y, Zhu Y, et al. Clinical Usefulness of Right Ventricle-Pulmonary Artery Coupling in Cardiovascular Disease. J Clin Med 2023;12:2526. [Crossref] [PubMed]
- Brener MI, Lurz P, Hausleiter J, et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J Am Coll Cardiol 2022;79:448-61. [Crossref] [PubMed]
- Ross C, Kumar R, Pelland-Marcotte MC, et al. Acute Management of High-Risk and Intermediate-Risk Pulmonary Embolism in Children: A Review. Chest 2022;161:791-802. [Crossref] [PubMed]
- Gerges C, Skoro-Sajer N, Lang IM. Right ventricle in acute and chronic pulmonary embolism (2013 Grover Conference series). Pulm Circ 2014;4:378-86. [Crossref] [PubMed]
- Falster C, Hellfritzsch M, Gaist TA, et al. Comparison of international guideline recommendations for the diagnosis of pulmonary embolism. Lancet Haematol 2023;10:e922-35. [Crossref] [PubMed]
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603. [Crossref] [PubMed]
- Luisada AA. Left ventricular failure and acute pulmonary edema. J Am Geriatr Soc 1953;1:331-9. [Crossref] [PubMed]
- Montgomery HE, Marshall M, Woolf I, et al. Pulmonary embolism as a cause of pulmonary oedema. Clinical Intensive Care 1995;6:184-8. [Crossref]
- Omori A, Toyota T, Arizono S, et al. Localized Pulmonary Edema Secondary to Pulmonary Embolism. JACC Case Rep 2024;29:102332. [Crossref] [PubMed]
- Jopart C, Hainaut P, Ghaye B. Pulmonary Edema: Consider an Unusual Suspect. J Belg Soc Radiol 2019;103:33. [Crossref] [PubMed]
- Zaidi AU, Hutchins KK, Rajpurkar M. Pulmonary Embolism in Children. Front Pediatr 2017;5:170. [Crossref] [PubMed]
- Grotberg JC, McDonald RK, Co IN. Point-of-Care Echocardiography in the Difficult-to-Image Patient in the ICU: A Narrative Review. Crit Care Explor 2024;6:e1035. [Crossref] [PubMed]
- Kanis J, Hall CL, Pike J, et al. Diagnostic accuracy of the D-dimer in children. Arch Dis Child 2018;103:832-4. [Crossref] [PubMed]
- Orsi FA, Angerami RN, Mazetto BM, et al. Reduced thrombin formation and excessive fibrinolysis are associated with bleeding complications in patients with dengue fever: a case-control study comparing dengue fever patients with and without bleeding manifestations. BMC Infect Dis 2013;13:350. [Crossref] [PubMed]
- Joob B, Wiwanitkit V. Platelet Count, D-Dimer, Component Therapy and Dengue Hemorrhagic Fever. Indian J Hematol Blood Transfus 2018;34:370-1. [Crossref] [PubMed]
- Caudron M, Holt T, Cuvelier G, et al. Pulmonary Thromboses in Pediatric Acute Respiratory Distress Syndrome. Respir Care 2019;64:209-16. [Crossref] [PubMed]
- McGuire WC, Sullivan L, Odish MF, et al. Management Strategies for Acute Pulmonary Embolism in the ICU. Chest 2024;166:1532-45. [Crossref] [PubMed]
Cite this article as: Chandra R, Chor YK. A rare complication in severe dengue: how point of care ultrasonography (POCUS) makes a difference?—a case report. J Emerg Crit Care Med 2025;9:16.


