
Feasibility of remimazolam use for sedation in critically ill patients with respiratory failure: a case series
Highlight box
Key findings
• We present a case series of five intensive care unit (ICU) patients with coronavirus disease 2019-related respiratory failure who received remimazolam for sedation. Remimazolam proved effective as a long-term sedative, with no observed adverse events.
What is known and what is new?
• Remimazolam, a fast-acting benzodiazepine, has been studied for procedural sedation and general anesthesia, but there is limited research on its use in the ICU.
• This case series provides preliminary evidence that remimazolam can maintain adequate sedation in critically ill patients with respiratory failure, including those on non-invasive and invasive respiratory support.
What is the implication, and what should change now?
• Remimazolam appears to be a safe and effective long-term sedative for critically ill ICU patients with respiratory failure. Larger randomized controlled trials are needed to confirm these findings and to clarify the role of remimazolam in the ICU setting.
Introduction
Remimazolam is a new fast-acting benzodiazepine, developed to provide rapid onset of sedation and quick recovery. Initially conceived for short procedural sedation, it was recently investigated in the setting of general anesthesia and intensive care unit (ICU) sedation. The pharmacodynamic profile is the result of a carboxylic ester linkage that is rapidly hydrolyzed by tissue esterases (1).
Its ultra-short acting property and organ-independent metabolism, along with the availability of an antidote, suggest its promising potential as a different choice to currently used hypnotics. Safety and effectiveness of remimazolam for endoscopic procedure was already established (2-5), even in high-risk American Society of Anesthesiology (ASA) patients (6).
In the last years, two multicenter, randomized, double-blind trials evaluated remimazolam efficacy for induction and maintenance of general anesthesia (7,8).
Favorable results encouraged further investigations in different settings. The critical scenario of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic revealed the need for alternative hypnotic solutions. The great demand for mechanical ventilation, secondary to a high rate of acute respiratory distress syndrome (ARDS), and the consequent increasing request for sedatives and analgesics, resulted in drug shortages (9).
In this context, it was reasonable to figure a possible application of remimazolam for ICU sedation, since it has the advantage to allow easier management of sedation-free intervals, hemodynamic stability, and a metabolism independent from liver and kidney which are frequently impaired in critically ill patients (10).
Currently, few published data are available on the use of remimazolam for sedation in ICU. We therefore decided to perform a study to evaluate the feasibility of remimazolam administration for ICU sedation. We present this article in accordance with the AME Case Series reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-123/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
We present a prospective case series of consecutive critically ill patients with coronavirus disease 2019 (COVID-19) who received intravenous remimazolam as sedative agent in ICU according to a compassionate use program in a tertiary referral center. This data collection was part of the COVID-BioB study approved by the Hospital Ethics Committee (protocol No. 34/int/2020) and registered on ClinicalTrials.gov (NCT04318366). Hospital organization and clinical management of COVID-19 patients at our center has been previously published (11-14).
Remimazolam was not approved by the Italian Drug Administration Agency (AIFA). Accordingly, we obtained Ethics Committee approval for compassionate use of the drug and all patients provided a written informed consent. For patients unable to provide consent, a deferred consent procedure was used in accordance with ethics committee recommendations.
Inclusion criteria
Patients who had ARDS admitted to the ICU and requiring sedation were screened for eligibility. Patients were enrolled if the treating clinicians agreed that use of a short-acting benzodiazepine to provide sedation might be beneficial. Exclusion criteria were: (I) use of neuromuscular blocking agents (NMBAs) in absence of processed electroencephalography (pEEG) depth-of-anesthesia monitoring; (II) contraindications to remimazolam use; and (III) uncontrolled hypertension.
Patient monitoring and management
All patients were monitored according to clinical needs, but at least with continuous 3- or 5-lead electrocardiography (ECG), continuous pulse oximetry, and invasive arterial pressure. Clinical sedation assessment was performed with the Richmond Agitation-Sedation Scale (RASS) as recommended by guidelines (15). Processed EEG (CONOX, Fresenius Kabi, Lake Zurich, IL, USA) was used in patients requiring deep sedation with or without neuromuscular blockade. Target level of sedation was individualized according to clinical needs. Before starting the study, medical and nursing staff were instructed on remimazolam preparation and management. Patient management, including need for additional monitoring and collection of laboratory parameters, was at discretion of attending clinicians. Use of additional non-opioids sedatives was discouraged. Use of opioids and NMBAs was at discretion of attending clinicians.
Remimazolam preparation, administration, and dosing
Remimazolam Besylate (Byfavo, Paion, Aachen, Germany) was diluted as 50 mg in 25 mL of normal saline, to obtain a final concentration of 2 mg/mL. Remimazolam was administered in a central or peripheral vein through a dedicated lumen with no other drugs or infusion running on the same lumen, to reduce the risk of inadvertent bolus administration or potential drug precipitation. The starting dose was 0.05 mg/kg/h (approximately 2 mL/h in a 70-kg patient), with a target dose of 0.25 mg/kg/h (dosage range, 0.05–2 mg/kg/h).
Data collection and follow-up
Trained investigators not involved in patients’ management collected the following data: baseline and demographic clinical characteristics including comorbidities and date of symptoms onset; hemodynamic and ventilatory variables [blood pressure, heart rate, need for and dose of inotropes and vasopressors, arterial partial oxygen tension (PaO2)/fraction of inspired oxygen (FiO2) ratio, lactate, ventilation mode and ventilator setting]; dose of remimazolam, RASS value, and quantium counsciousness index (qCON) value in case of use of pEEG monitoring. Hemodynamic and ventilatory variables were collected at start of remimazolam infusion, at 1 hour and at 8 hours after remimazolam administration. We also collected data on adverse events related to remimazolam administration and patient’s outcome (in-hospital mortality).
Study outcomes
For this study, patients were followed up until death or hospital discharge. The primary outcome was feasibility of remimazolam administration in ICU setting, defined as maintenance of target sedation level without need for additional non-opioids hypnotics. Secondary outcomes included in-hospital mortality and adverse events related to remimazolam use.
Patients characteristics
A total of five ARDS male patients, satisfying inclusion criteria, were enrolled to receive remimazolam in February 2021. A detailed description of each single patient, including main personal medical history, hospital access, and the following in-hospital course is reported in supplementary material (Appendix 1). Three patients (cases 1–3) maintained spontaneous breathing during sedation. The remaining two subjects (cases 4 and 5) were already intubated and mechanically ventilated under NMBA administration, when the remimazolam infusion was started. Demographic data (age and comorbidities), basal laboratory values, severity of illness score, ventilation modality, patients’ position, duration of infusion and dosage of remimazolam are reported in Tables 1,2.
Table 1
| Patient number [age in years] | Ventilatory support | Position | Infusion duration | Dosage (mg/kg/h) | Drug previously administered | Vasoactive agent |
|---|---|---|---|---|---|---|
| Patient 1 [55] | HFNC-SB | Prone | 7 hours 55 minutes. Night hours | 0.05 | Remifentanil | No |
| Patient 2 [72] | HFNC-SB | Left lateral | 7 hours 25 minutes. Night hours | 0.05 | Remifentanil | No |
| Patient 3 [68] | VM-SB | Supine | 15 hours (2 days). Night hours | 0.09 | Remifentanil | No |
| Patient 4 [64] | Bipap-OTI-IMV | Supine | 61 hours 35 minutes. Continuous infusion | 0.05–2 | Propofol, midazolam, fentanyl | Norepinephrine |
| Patient 5 [67] | Bipap-OTI-IMV | Supine | 8 hours 5 minutes. Night hours | 0.05–0.4 | Propofol, midazolam, fentanyl | Norepinephrine |
Sedative regimen adopted before remimazolam use. All the hypnotic agents used before were stopped after starting remimazolam administration. In patient 4, the use of remimazolam allowed a progressive reduction in norepinephrine administration (from 0.25 to 0.1 mcg/kg/min in 8 hours). All the patients were male. Bipap, bilevel positive airway pressure; HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation; OTI, orotracheal intubation; SB, spontaneous breathing; VM, venturi mask.
Table 2
| Patient number | Comorbidities | SOFA score | Hemoglobin (g/dL) | Creatinine (mg/dL) | AST/ALT (U/L) |
|---|---|---|---|---|---|
| Patient 1 | NRC | 2 | 13.4 | 0.87 | 37/92 |
| Patient 2 | AH-bipolar Sdr | 3 | 9.9 | 1.46 | 10/22 |
| Patient 3 | Bipolar Sdr-IC-diabetes-COPD-parkinsonism-RRT | 5 | 10.2 | 1.38 | 52/41 |
| Patient 4 | Epilepsy-AH-20% ejection fraction | 9 | 9.2 | 0.96 | 300/126 |
| Patient 5 | Diabetes-AH-AR | 10 | 9.1 | 1.33 | 220/242 |
AH, arterial hypertension; ALT, alanine transaminase; AR, arthritis rheumatoid; AST, aspartate transaminase; COPD, chronic obstructive pulmonary disease; IC, ischemic cardiopathy; NRC, non-relevant comorbidities; RRT, renal replacement therapy; Sdr, syndrome; SOFA, Sequential Organ Failure Assessment.
Remimazolam administration regimen and concomitant sedative and vasoactive therapies
Drug dosage was modified according to clinical response. Median duration of administration was 8 hours and dosage of continuous infusion ranged between 0.05 and 2 mg/kg/h (Table 1). Specifically, we tried to obtain a moderate sedation in patients in spontaneous breathing (cases 1–3; target moderate sedation, −3 score according to RASS classification). On the contrary, we maintained a qCON value on CONOX between 40 and 60 in intubated patients, receiving muscle-relaxants under mechanical ventilation (cases 4 and 5). Sedative utilized before remimazolam administration are reported in Table 1. Two patients were hemodynamically unstable and managed with a continuous infusion of norepinephrine; in one of these two patients, the adoption of remimazolam allowed a progressive reduction in the dosage of norepinephrine (from 0.25 to 0.1 mcg/kg/min after 8 hours) (Table 1).
Primary and secondary outcomes
Considering the primary outcome (feasibility of remimazolam administration as single-drug sedative regimen), remimazolam was effective in maintaining desired target sedation without the need of using rescue hypnotic agents. In one of the invasively ventilated patients, remimazolam allowed to halve the concomitant dose of propofol but not to suspend its administration.
We did not observe modifications in respiratory parameters in the three patients in spontaneous breathing: oxygen saturation, arterial oxygen, pH, lactate, and arterial carbon dioxide did not present substantial modifications according to continuous monitoring and results from serial blood gas analyses (Table S1, Figures S1-S4).
As secondary outcomes, we did not observe adverse events related to remimazolam administration.
We report a substantial hemodynamic stability in all treated patients. Heart rate, mean arterial pressure and pH at different time-points are illustrated in Figures 1-3 and in supplementary material (Table S2, Figures S5,S6).
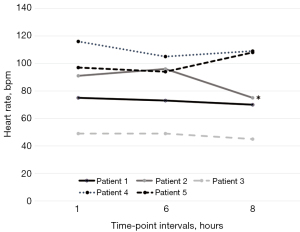
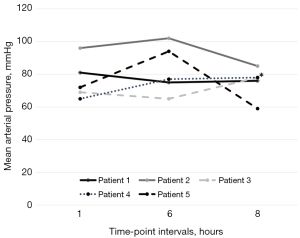
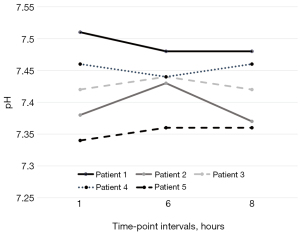
The interruption of sedation was not associated to cognitive dysfunction and agitation, nor to the observation of modifications in RASS values compared to previous days (spontaneous breathing patients). Furthermore, remimazolam infusion was not associated to alterations in blood tests including liver and renal function tests, blood count, and myonecrosis markers at 48 hours after interruption of drug administration. No other adverse events were observed.
Discussion
Key findings
In this preliminary case series, we found that remimazolam administration was feasible as a single-drug hypnotic regimen in achieving adequate sedation levels in critically ill patients with respiratory failure, without showing adverse events.
Relationship to previous studies
In 1985, Dundee wrote that the ideal hypnotic agent should be water soluble, non-irritating, with no-analgesic action in order to reduce as much as possible cardiovascular and respiratory depression. Furthermore, he specified that a slight delay in onset could be acceptable if predictable (16).
In a narrative review, Sneyd et al. evaluated the status of perioperative hypnotics, underlying how the number of potential drugs is quite limited and recently further reduced by the withdrawn and the near disappearance of thiopental respectively in US and Europe (17). In this article, the authors underlined how remimazolam presents many of the characteristics of an ideal hypnotic agent, including rapid onset, a formulation not supporting bacterial growth, no pain on injection, no metabolite active, flumazenil as reversal agent, no adrenocortical depression and minimal hypotension. However, they also reported the absence of data on drug’s offset for prolonged infusion. In the last years, scientific evidence suggesting a role for remimazolam as possible alternative to traditional hypnotic agent has grown increasingly. Several randomized controlled trials (RCTs) in the setting of sedation for colonscopy highlighted that the use of this new benzodiazepine was associated to no pain on injection and to fewer episodes of hypotension and respiratory depression in contrast to propofol-treated patients; a non-inferior recovery profile was also maintained (18,19).
A following meta-analysis and systematic review confirmed the higher success procedure rate, a fast recovery, and an adequate safety profile for the use of remimazolam during procedural sedation (20).
Similiar findings were reported also for patients undergoing general anesthesia (7,8). Considering all these positive data, Van de Velde et al. suggested in a dedicated editorial that the quest for the holy grail for procedural sedation could be over (21).
On the contrary, evidence supporting the use of remimazolam in ICU are still scarce (22).
In 2013, the ICU at Hirosaki University School of Medicine and Hospital realized a multicenter randomized phase II study. The trial was registered with protocol number ONO-2745-04 and studied safety and effectiveness of three different dosage of Remimazolam for sedation in postoperative patients requiring invasive ventilation. Besides the above-mentioned ONO trial, there are few studies with limited sample size that studied this new benzodiazepine in the setting of ICU. In a recent pilot trial, 60 patients admitted to ICU were randomized to receive remimazolam or propofol to maintain mild-moderate sedation under long-term mechanical ventilation (23).
The median duration of study drug infusion was not statistically different in two groups (55 hours remimazolam versus 41 hours propofol). Both hypnotic agents were equally effective in reaching the target RASS range, similarly no differences were observed for safety outcomes (ventilator free days at day 7, length of ICU stay, 28-day mortality, adverse events).
Compared to these studies, our work is the first assessing the use of remimazolam specifically in patients with respiratory failure, for whom sedation and control of respiratory drive may be particularly important (24).
Furthermore, this is the first study investigating the role of remimazolam in patients with respiratory failure not receiving invasive ventilation, a category of patients particularly vulnerable to adverse events from inadequate (either excessive or insufficient) sedation.
Implications of study findings
Our case series confirmed the positive results coming from the pilot study by Tang et al. and Yao et al. (23,25), about the efficacy and safety in using remimazolam for long-term ICU sedation. The reduction of norepinephrine dosage in one case seems to support the use of this benzodiazepine in hemodynamically unstable patients. Furthermore, remimazolam was successfully adopted in non-intubated patients, in spontaneous breathing and managed with non-invasive ventilation (NIV). Future large, randomized studies could focus on these peculiar aspects in both adult and pediatric patients (26).
Need for additional data on different sedative agents for both perioperative and critically ill patients are particularly important, as there is some evidence that specific drugs may affect survival when used for induction and/or maintenance of sedation (27,28).
Furthermore, despite these positive preliminary results, prolonged infusion of remimazolam is still associated to several concerns. First, in the ONO trial, analyzing blood samples at 24 hours or more after starting infusion, a higher concentration of remimazolam was observed than expected (20). Moreover, remimazolam presented recovery times superior to propofol in studies performed during general anesthesia and procedural sedation. This issue needs to be further evaluated and clarified in the context of long-term prolonged infusion. Additionally, several evidence suggested that benzodiazepine administration (in particular, when continuous infusion is adopted), can increase the risk for delirium in critically ill adults (29).
Future studies performed in the ICU setting will have to clarify this topic. Finally, considering clinical aspects requiring additional investigations, anaphylactic issues have been reported with the use of remimazolam, with 10 cases in Japan and South Korea (30).
Collectively, our data, although deriving from a small population, provide additional clues on potential fields of application of remimazolam in ICU, and generally reinforce the message of safety. Interestingly, two different types of remimazolam salts (besylate and tosylate) exist in clinical practice, raising the issue if the results obtained with the two different formulations can be considered totally comparable. Of note, the issue of comparing doses of different salt formulations of the same drug has been recently raised also for norepinephrine. This issue will also be subject to future investigations (31-34).
Strengths and limitations
Our study presents several weaknesses, including a small sample size, the absence of a control group, and no pharmacokinetic analysis. The limited number of enrolled patients was due to the authorization of using remimazolam only as compassionate drug and to the shortage, also, for this hypnotic during the COVID-19 pandemic. In our case series, remimazolam confirmed its efficacy and safety profile also in critically ill patients and in one case its infusion allowed to reduce the dosage of norepinephrine. Notably, to the best of our knowledge, for the first time, we tested this new benzodiazepine for prolonged sedation in ICU patients managed with NIV in spontaneous breathing. In all these cases, remimazolam was effective in reaching moderate sedation without respiratory and cardiac adverse events. Due to the limited number of enrolled patients, the positive findings of this case series need to be confirmed by large RCTs.
Conclusions
In conclusion, our case series confirmed the effectiveness and safety of remimazolam as long-term sedative agent in critically ill adult patients. Future large, randomized trials are necessary to validate the role of remimazolam in the ICU setting, analyzing possible side effects related to long-term infusion, advantages compared to traditional hypnotics and fields of use (long-term infusion for patients under mechanical ventilation, short sedation during ICU invasive procedures).
Acknowledgments
The authors thank all the personnel of San Raffaele Scientific Institute for the dedication to these patients and for the support in data collection.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-123/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-123/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-123/coif). A.B. serves as an unpaid editorial board member of Journal of Emergency and Critical Care Medicine from January 2023 to December 2026. S.T. reports honoraria for lecturing from Viatris. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The data collection was part of the COVID-BioB study approved by the Hospital Ethics Committee (protocol No. 34/int/2020) and registered on ClinicalTrials.gov (NCT04318366). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology 2007;107:60-6. [Crossref] [PubMed]
- Pastis NJ, Yarmus LB, Schippers F, et al. Safety and Efficacy of Remimazolam Compared With Placebo and Midazolam for Moderate Sedation During Bronchoscopy. Chest 2019;155:137-46. [Crossref] [PubMed]
- Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 2018;88:427-437.e6. [Crossref] [PubMed]
- Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res 2020;12:4594-603. [PubMed]
- Deng L, Zhou R, Jiang W, et al. Efficacy and safety of remimazolam besylate in patients with obesity undergoing painless colonoscopy: a prospective, double-blind, randomized controlled trial. Signa Vitae 2024;20:76-85.
- Rex DK, Bhandari R, Lorch DG, et al. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis 2021;53:94-101. [Crossref] [PubMed]
- Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth 2020;34:543-53. [Crossref] [PubMed]
- Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth 2020;34:491-501. [Crossref] [PubMed]
- Ammar MA, Sacha GL, Welch SC, et al. Sedation, Analgesia, and Paralysis in COVID-19 Patients in the Setting of Drug Shortages. J Intensive Care Med 2021;36:157-74. [Crossref] [PubMed]
- Goudra BG, Singh PM. Remimazolam: The future of its sedative potential. Saudi J Anaesth 2014;8:388-91. [Crossref] [PubMed]
- Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Ramirez GA, Bozzolo EP, Castelli E, et al. Continuous positive airway pressure and pronation outside the Intensive Care Unit in COVID-19 acute respiratory distress syndrome. Minerva Med 2022;113:281-90. [Crossref] [PubMed]
- Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc 2020;22:200-11. [Crossref] [PubMed]
- Guzzo F, Lombardi G, Tozzi M, et al. Feasibility, safety and efficacy of COVID-19 severe acute respiratory distress syndrome management without invasive mechanical ventilation. Minerva Anestesiol 2023;89:1013-21. [Crossref] [PubMed]
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Dundee JW. Intravenous anaesthesia and the need for new agents. Postgrad Med J 1985;61:3-6. [PubMed]
- Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth 2021;127:41-55. [Crossref] [PubMed]
- Yao Y, Guan J, Liu L, et al. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomised, double-blind trial. Eur J Anaesthesiol 2022;39:911-7. [Crossref] [PubMed]
- Guo L, Liu T, Zhang Y, et al. Effect of remimazolam versus propofol sedation on the quality of recovery after colonoscopy: a randomised, controlled, noninferiority trial. Eur J Anaesthesiol 2022;39:953-5. [Crossref] [PubMed]
- Tang Y, Yang X, Yu Y, et al. Remimazolam versus traditional sedatives for procedural sedation: a systematic review and meta-analysis of efficacy and safety outcomes. Minerva Anestesiol 2022;88:939-49. [Crossref] [PubMed]
- Van de Velde M, Hansen TG. Remimazolam: Is the quest for the holy grail of sedation during diagnostic or endoscopic interventions over? Eur J Anaesthesiol 2022;39:909-10. [Crossref] [PubMed]
- Choi HR, Song IA. Review of remimazolam and sedatives in the intensive care unit. Acute Crit Care 2022;37:151-8. [Crossref] [PubMed]
- Tang Y, Yang X, Yu Y, et al. Remimazolam besylate versus propofol for long-term sedation during invasive mechanical ventilation: a pilot study. Crit Care 2022;26:279. [Crossref] [PubMed]
- Spinelli E, Mauri T, Beitler JR, et al. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 2020;46:606-18. [Crossref] [PubMed]
- Yao Z, Liao Z, Li G, et al. Remimazolam tosylate's long-term sedative properties in ICU patients on mechanical ventilation: effectiveness and safety. Eur J Med Res 2023;28:452. [Crossref] [PubMed]
- Budic I, Marjanovic V, Djordjevic I, et al. Procedural sedation and analgesia in the pediatric intensive care unit. Signa Vitae 2023;19:38-46.
- Kotani Y, Pruna A, Turi S, et al. Propofol and survival: an updated meta-analysis of randomized clinical trials. Crit Care 2023;27:139. [Crossref] [PubMed]
- Kotani Y, Piersanti G, Maiucci G, et al. Etomidate as an induction agent for endotracheal intubation in critically ill patients: A meta-analysis of randomized trials. J Crit Care 2023;77:154317. [Crossref] [PubMed]
- Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med 2015;41:2130-7. [Crossref] [PubMed]
- Cinotti R. An update on remimazolam and anaphylaxis. Eur J Anaesthesiol 2023;40:153-4. [Crossref] [PubMed]
- Salvati S, D'Andria Ursoleo J, Belletti A, et al. Norepinephrine Salt Formulations and Risk of Therapeutic Error: Results of a National Survey. J Cardiothorac Vasc Anesth 2024;38:2624-9. [Crossref] [PubMed]
- Kotani Y, Belletti A, D'Andria Ursoleo J, et al. Norepinephrine Dose Should Be Reported as Base Equivalence in Clinical Research Manuscripts. J Cardiothorac Vasc Anesth 2023;37:1523-4. [Crossref] [PubMed]
- Leone M, Goyer I, Levy B, et al. Dose of norepinephrine: the devil is in the details. Intensive Care Med 2022;48:638-40. [Crossref] [PubMed]
- Wieruszewski PM, Leone M, Kaas-Hansen BS, et al. Position Paper on the Reporting of Norepinephrine Formulations in Critical Care from the Society of Critical Care Medicine and European Society of Intensive Care Medicine Joint Task Force. Crit Care Med 2024;52:521-30. [Crossref] [PubMed]
Cite this article as: Turi S, Belletti A, Marmiere M, Faustini C, Campugiani A, D’Amico F, Marchetti C, Di Piazza M, Maimeri N, Beretta L; the COVID-BioB Study Group. Feasibility of remimazolam use for sedation in critically ill patients with respiratory failure: a case series. J Emerg Crit Care Med 2025;9:14.


