
Hyperlactatemia in critically ill patients with COVID-19 is associated with increased mortality
Highlight box
Key findings
• Serum lactate is a biomarker closely associated with morbidity and mortality in critically ill patients.
What is known and what is new?
• Hyperlactatemia in critically ill patients with severe coronavirus disease 2019 (COVID-19) admitted to the intensive care unit is associated with a higher risk of death.
• It is noteworthy that the association between hyperlactatemia and increased mortality persists even with only mildly elevated lactate levels.
What is the implication, and what should change now?
• Levels as low as 1.6 mmol/L should raise the alert for earlier action in the resuscitation of patients with severe COVID-19.
Introduction
Multiple-organ dysfunction is closely related to the hypoperfusion and inflammation associated with sepsis, and hyperlactatemia signals both mechanisms. Serum lactate is a biomarker closely associated with morbidity and mortality in critically ill patients (1-4). Hyperlactatemia raises the mortality rate of septic patients by up to 5 times compared to the absence of hyperlactatemia (5). Particularly in the initial presentation of a septic condition, hyperlactatemia is related to hypoperfusion (1,3,6,7). In addition, blood lactate increases via nonhypoxic mechanisms (8,9). In any context of critical illness, normalization of or decreases in lactate levels after the initiation of treatment are associated with a better outcome (3,4,10,11).
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although most patients present with mild symptoms, some may progress to severe acute respiratory syndrome (SARS), multiple-organ failure, and death (12). COVID-19 does not usually present with septic shock, but among nonsurvivors, this is one of the most frequent complications (13). Patients with COVID-19 seem to make less lactate than patients with bacterial septic shock (14), but it still seems to be an important prognostic marker (15).
Severe COVID-19 is associated with systemic dysfunction, and monitoring the blood lactate concentration is expected to be useful regardless of the presence of circulatory shock. The objective of this study was to investigate the association between hyperlactatemia and in-hospital mortality in critically ill patients with COVID-19 admitted to the intensive care unit (ICU), as well as to determine the optimal lactate cutoff value for predicting 28-day mortality. We present this article in accordance with the STROBE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-116/rc).
Methods
Design
This observational and retrospective study was conducted in the ICU of the Hospital de Clínicas de Porto Alegre, which is a tertiary-level teaching hospital. The ICU operates in a closed model and is fully covered by certified intensivists.
Selection of patients
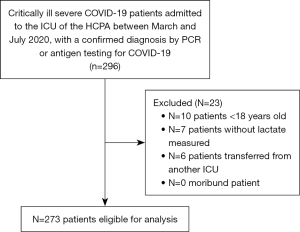
This study included patients admitted to the ICU between March and July 2020. The inclusion criteria were patients aged 18 years or older who were diagnosed with SARS-CoV-2 by real-time polymerase chain reaction (PCR) or antigen detection through immunochromatographic examination, only those whose ICU admission was due to severe COVID-19 were included. All ICU admissions were unscheduled, and data from only the initial ICU admission were included in the analysis, with any subsequent readmissions to the ICU being excluded. Patients without serum lactate measurements, patients who were considered moribund (patients experiencing multiple organ failure, severe hypoperfusion, or irreversible conditions where any attempt at curative treatment is deemed futile), and whose treatment was discontinued within 48 hours for patients whose therapy or life support was considered futile following a clinical discussion between intensivists and the palliative care team, and those who were transferred from other ICUs were excluded from the study (Figure 1).
Data collection
Clinical and sociodemographic data were obtained by reviewing the electronic medical records of patients after their ICU admission. The variables collected included age, sex, blood pressure, need for organ support [use of MV, need for vasopressors on the 1st and 3rd days, need for renal replacement therapy (RRT)], PaO2/FiO2 ratio on the 1st and 3rd days, length of stay in the hospital, and mortality rate. Disease severity was assessed on the Simplified Acute Physiology Score 3 (SAPS-3) (16). Arterial lactatemia was measured daily during the first 3 days of ICU admission, and for each day, the highest arterial lactate value recorded was included in the analysis. The collection times were as follows—day 1: within the first 24 hours of ICU admission; day 2: between 24 and 48 hours after ICU admission; day 3: between 48 and 72 hours after ICU admission.
The collected blood samples were kept on ice and measured for blood lactate immediately. An ABL800 Flex Analyzer device (Radiometer Medical ApS, Åkadevej 21, DK-2700 Brønshøj, Denmark) was used to perform blood gas analysis and measure arterial lactate. The normal reference range for lactate levels in the Hospital de Clínicas de Porto Alegre laboratory is 0.5 to 2.0 mmol/L. The patients were divided into two groups, serum lactate <2 mmol/L and serum lactate ≥2 mmol/L, for comparison of variables of interest. We selected this lactate level based on the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3, 2016), a landmark study that redefined septic shock to include serum lactate levels >2 mmol/L as a key component, and the Surviving Sepsis Campaign [2016], which revised the threshold for concern by lowering it from 4 to 2 mmol/L.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed data or as median [interquartile range (IQR)] for non-normally distributed data. They were compared using the Wilcoxon-Mann-Whitney test, as the assumption of normality was violated. Categorical variables are presented as n (%) and were compared using the χ2 test or Fisher’s exact test, as appropriate. Receiver operating characteristic (ROC) curves were drawn to evaluate the global discriminative power of the lactate concentration in the sample by calculating the area under the ROC curve (AUC), and Youden’s index (combination of sensitivity and specificity) was used to determine the optimal cutoff point for the lactate concentration. To measure the effect of lactate on mortality, we used a Poisson multiple regression model with robust variances, adjusting for known risk factors for mortality in critically ill patients admitted to the ICU, such as age, SAPS-3 score (prognostic variable), and the use of organ support (MV, the use of vasopressors, RRT). Thus, we obtained estimates of the relative risk (RR) and 95% confidence intervals (CIs) for high lactate levels. Values of P<0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS v. 25.0 (IBM-SPSS, IBM Corp., Armonk, New York, USA) and JAMOVI v. 2.3.21 (https://www.jamovi.org). The sample size was calculated based on anticipated mortality rates in critically ill patients with sepsis, a condition with presumed pathophysiological similarities to severe COVID-19. Previous studies have reported mortality rates ranging from 30% to 60% in patients with sepsis in ICU (1,5). To detect a RR of 1.5 with a power of 80% and a significance level of 5%, a minimum sample size of 250 patients was required. The study ultimately included 273 patients, surpassing the calculated sample size and ensuring adequate statistical power. The sample size calculation was performed using the PSS Health tool, version 0.3.1.
Ethical considerations
This study was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre (Certificate of Presentation and Ethical Assessment No. 51976521.8.0000.5327, with Substantial Opinion No. 5.080.112). A review of the hospital records of patients admitted to the ICU was authorized, with the waiver of free and informed consent. The study adhered to the Declaration of Helsinki (as revised in 2013), and the anonymity of patients was preserved.
Results
We evaluated 296 patients who were diagnosed with severe COVID-19 and admitted to the ICU between March and July 2020; 23 patients were excluded, leaving 273 patients for the final analysis (Figure 1). The mean age was 58±13 years, with 116 female patients (42.5%). Of the 273 patients, 115 did not survive (42%). The median SAPS-3 was 59 (IQR, 49–70). Two hundred eleven patients required MV (77%). Vasopressors were given to 142 (52%) and 143 (52%) patients on the 1st and 3rd days, respectively. Seventy-five patients (28%) received RRT. The median hospital stay for the study population was 22 days (IQR, 13–34 days).
In Table 1, we report the clinical characteristics of the entire study population broken down by the presence of hyperlactatemia (lactate ≥2 mmol/L). When comparing patients with and without hyperlactatemia, the groups did not differ in male/female ratio; the rate of active neoplasia, heart disease, or systemic arterial hypertension; C-reactive protein or bilirubin level; or PaO2/FiO2 ratio on the 3rd day. Patients with hyperlactatemia were older, more ill (SAPS-3 score), more often had type 2 diabetes (DM2), had a worse glomerular filtration rate, had lower blood pressure, and had worse pulmonary gas exchange (lower PaO2/FiO2 ratio) in the first 24 hours.
Table 1
| Variables | Total | Lactate <2 mmol/L | Lactate ≥2 mmol/L | P |
|---|---|---|---|---|
| Patients | 273 | 194 (71.0) | 79 (29.0) | |
| Age (years) | 58.3±13.5 | 57.3±13.2 | 61.1±13.6 | 0.03* |
| Sex | ||||
| Male | 157 (57.5) | 114 (58.8) | 43 (54.4) | 0.51 |
| Female | 116 (42.5) | 80 (41.2) | 36 (45.6) | |
| SAPS-3 | 59 [49–70] | 55.5 [47–65] | 67 [60–79] | <0.001* |
| Comorbidities | ||||
| DM2 | 92 (33.7) | 54 (27.8) | 38 (48.1) | 0.03* |
| Heart disease | 47 (17.2) | 26 (13.4) | 21 (26.6) | 0.06 |
| SH | 160 (58.6) | 106 (54.6) | 54 (68.4) | 0.30 |
| Neoplasm | 17 (6.2) | 8 (4.1) | 9 (11.4) | 0.08 |
| MAP on the 1st day (mmHg) | 80 [71–90] | 84.3 [75–95] | 73 [67.5–84.5] | 0.001* |
| MAP on the 3rd day (mmHg) | 79 [71–90] | 81.5 [73.3–90] | 75 [68.5–81] | 0.001* |
| PaO2/FiO2 1st day | 180 [118–258] | 187 [124–265] | 168 [100–238] | 0.02* |
| PaO2/FiO2 3rd day | 201 [145–269] | 204 [147–257] | 186 [142–281] | 0.82 |
| CRP (mg/L) | 145 [85–220] | 144 [85.5–213] | 150 [85.8–225] | 0.72 |
| Creatinine 1st day (mg/L) | 1.04 [0.78–1.7] | 1.00 [0.76–1.6] | 1.20 [0.86–2.17] | 0.01* |
| Creatinine 3rd day (mg/L) | 1.0 [0.74–1.81] | 0.89 [0.72–1.60] | 1.31 [0.9–1.88] | 0.003* |
| Bilirubin 1st day (mg/dL) | 0.5 [0.30–0.80] | 0.45 [0.38–0.80] | 0.5 [0.30–0.7] | 0.98 |
| Bilirubin 3rd day (mg/dL) | 0.65 [0.4–1.02] | 0.60 [0.40–1.00] | 0.85 [0.48–1.73] | 0.21 |
| Deaths | 115 (42.1) | 60 (30.9) | 55 (69.6) | <0.001* |
| Vasopressor 1st day | 142 (52.0) | 88 (45.4) | 54 (68.4) | <0.001* |
| Vasopressor 3rd day | 143 (52.4) | 88 (45.4) | 55 (69.6) | <0.001* |
| Need for RRT | 75 (27.5) | 49 (25.3) | 26 (32.9) | 0.20 |
| MV (days) | 10 [2–23] | 8 [0–20] | 13 [7–25] | 0.008* |
| MV | 211 (77.3) | 137 (70.6) | 74 (93.7) | <0.001* |
Data are presented as mean ± SD, n (%), or median [IQR]. *, below the preset threshold of P<0.05. SAPS-3, Simplified Acute Physiology Score 3; DM2, type 2 diabetes; SH, systemic hypertension; MAP, mean arterial pressure; PaO2/FiO2, ratio of arterial oxygen pressure (PaO2) to the fraction of inspired oxygen (FiO2); CRP, C-reactive protein; RRT, renal replacement therapy; MV, mechanical ventilation; SD, standard deviation; IQR, interquartile range.
Hyperlactatemic patients had a greater need for vasoactive drugs on the 1st and 3rd days and received more ventilatory support, but no greater need for RRT. Mortality was significantly higher in patients with lactate ≥2 mmol/L [69.6% (55/79) vs. 30.9% (60/194)].
Nonsurvivors exhibited significantly higher peak serum lactate concentrations compared to survivors, highlighting the association between elevated lactate levels and worse outcomes (Table 2).
Table 2
| Serum lactate (mmol/L) | Survivors | Nonsurvivors | P |
|---|---|---|---|
| 1st day | 1.4 (1.1–1.5) | 1.9 (1.5–2.56) | <0.001* |
| 2nd day | 1.2 (1.0–1.6) | 1.5 (1.2–1.9) | <0.001* |
| 3rd day | 1.2 (1.0–1.5) | 1.7 (1.5–2.0) | <0.001* |
Data are presented as median (IQR). *, below the preset threshold of P<0.05. IQR, interquartile range.
Figure 2 shows the ROC curves and the respective AUC-ROCs of lactate on the 1st, 2nd, and 3rd days, with their RRs for in-hospital death. The presence of hyperlactatemia (lactate ≥2 mmol/L) was associated with mortality during the first 3 days in the hospital. The AUC-ROC in the first 24 h was 0.73 (95% CI: 0.67–0.79), with a RR of 2.24 (95% CI: 1.73–2.90; P<0.001); on the 2nd day, it was 0.69 (95% CI: 0.61–0.77), with a RR of 1.66 (95% CI: 1.19–2.33; P=0.002); and on the 3rd day, it was 0.78 (95% CI: 0.70–0.85), with a RR of 2.36 (95% CI: 1.77–3.16; P≤0.001).
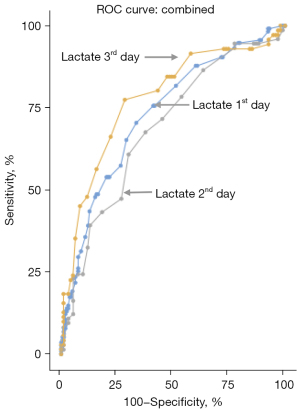
Table 3 provides an overview of how lactate levels performed as a diagnostic tool for predicting in-hospital mortality during the first three days of ICU admission. On day 1, a lactate cutoff of 1.6 mmol/L offered the best balance between sensitivity (70.4%) and specificity (66.5%) for identifying patients at higher risk of mortality. While the sensitivity and specificity of lactate cutoffs slightly declined on days 2 and 3, their predictive value remained clinically significant. Notably, the highest diagnostic accuracy, reflected by the AUC (0.78), was achieved on day 3.
Table 3
| Cutoff point | AUC-ROC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Yi |
|---|---|---|---|---|---|---|
| 1st day lactate | 0.73 | |||||
| 1.50 | 75.6 | 58.9 | 57.2 | 76.9 | 0.345 | |
| 1.60 | 70.4 | 66.5 | 60.5 | 75.5 | 0.369 | |
| 1.70 | 65.2 | 70.9 | 62.00 | 73.7 | 0.361 | |
| 1.80 | 57.4 | 73.4 | 61.1 | 70.3 | 0.308 | |
| 1.90 | 53.9 | 79.7 | 66.0 | 70.4 | 0.337 | |
| 2.00 | 48 | 84.5 | 69.6 | 69.0 | 0.326 | |
| 2nd day lactate | 0.69 | |||||
| 1.50 | 60.8 | 69.9 | 61.6 | 69.1 | 0.307 | |
| 1.60 | 47.3 | 73.1 | 58.3 | 63.5 | 0.204 | |
| 1.70 | 43.2 | 81.7 | 65.3 | 64.4 | 0.249 | |
| 1.80 | 39.2 | 87.1 | 70.7 | 64.2 | 0.263 | |
| 1.90 | 32.4 | 88.2 | 68.5 | 62.1 | 0.206 | |
| 2.00 | 24.3 | 90.3 | 66.7 | 60.0 | 0.146 | |
| 3rd day lactate | 0.78 | |||||
| 1.50 | 77.4 | 71.6 | 67.0 | 80.9 | 0.490 | |
| 1.60 | 66.2 | 77.9 | 69.1 | 75.5 | 0.441 | |
| 1.70 | 56.3 | 84.2 | 72.7 | 72.1 | 0.405 | |
| 1.80 | 47.9 | 88.4 | 75.5 | 69.4 | 0.363 | |
| 1.90 | 45.1 | 91.6 | 80 | 69.0 | 0.366 | |
| 2.00 | 35.2 | 93.7 | 80.6 | 65.9 | 0.288 |
ROC, receiver operating characteristic; AUC-ROC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value; Yi, Youden’s index.
A Poisson regression model with robust variances was built to determine whether lactate level (in the first 24 hours) was independently associated with in-hospital mortality in ICU patients with COVID-19 (Table 4). After adjusting for the effects of age, SAPS-3 score, MV, vasopressor use, and RRT, the RR of lactate ≥1.6 mmol/L was 1.25 (95% CI: 1.13–1.39), and that of lactate ≥2 mmol/L was 1.64 (95% CI: 1.29–2.09, P<0.001).
Table 4
| Variables | RR | Confidence Interval | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Lactate ≥1.6 mmol/L | 1.25 | 1.13 | 1.39 | <0.001* |
| Age | 1.01 | 1.00 | 4.02 | <0.001* |
| SAPS-3 | 1.00 | 0.99 | 1.01 | 0.69 |
| MV | 1.62 | 1.32 | 1.93 | 0.001* |
| RRT | 1.25 | 1.12 | 1.41 | <0.001* |
| Vasopressor | 1.17 | 1.04 | 1.30 | 0.007* |
| Lactate ≥2 mmol/L | 1.64 | 1.29 | 2.09 | <0.001* |
| Age | 1.02 | 1.01 | 1.03 | <0.001* |
| SAPS-3 | 1.00 | 0.99 | 1.01 | 0.69 |
| MV | 2.81 | 1.56 | 6.16 | 0.001* |
| RRT | 1.69 | 1.33 | 2.14 | <0.01* |
| Vasopressor | 1.43 | 1.06 | 1.93 | 0.02* |
a, only the lactate values on the 1st day met the criteria that allowed for this regression model. *, below the preset threshold of P<0.05. RR, relative risk; SAPS-3, Simplified Acute Physiology Score 3; MV, mechanical ventilation; RRT, renal replacement therapy.
Discussion
Our study highlights that hyperlactatemia is closely associated with in-hospital mortality among critically ill patients with COVID-19 admitted to the ICU. While lactate levels ≥2 mmol/L are well-recognized as markers of poor outcomes, we observed that COVID-19 patients did not typically present with exceedingly high lactate concentrations. This observation led us to investigate a more precise cutoff point for identifying those at the greatest risk. We found that even relatively modest elevations in lactate (≥1.6 mmol/L) were independently associated with increased mortality. Among the 273 patients analyzed, the overall in-hospital mortality rate was 42%, reflecting the severe impact of critical illness in this cohort. These findings align with previous studies that establish lactate as a valuable prognostic tool in critically ill populations, reinforcing its role in early risk stratification and guiding clinical interventions. Furthermore, lactate levels ≥2 mmol/L were linked to an even greater RR of mortality, underscoring the need for vigilant monitoring of lactate dynamics in these patients.
A concentration of lactate ≥1.6 mmol/L, according to Youden’s index (0.369), was the cutoff point that performed best at identifying patients who would die on the 1st day of hospitalization. This association between lactate ≥1.6 mmol/L and death is particularly interesting considering that lactate in the first 24 hours is an independent factor associated with death. Our study showed that hyperlactatemia, even fairly low hyperlactatemia, predicted death in the hospital, in line with other studies in patients with COVID-19 (17-21). A blood lactate concentration ≥1.6 mmol/L as a predictor of death is near the 1.55 mmol/L cutoff found by Wendel Garcia et al. (17), which increased the risk of death at 28 days with a hazard ratio =1.59 (95% CI: 1.04–2.44). Vassiliou et al. evaluated 45 patients and reported that lactate ≥1.85 mmol/L showed a sensitivity of 64% and a specificity of 79.4% for predicting 28-day mortality (20). Finally, a systematic review found an odds ratio (OR) of 3.66 (95% CI: 2.26–5.94) for a lactate concentration ≥1.5–2.2 mmol/L for death in COVID-19 patients (18).
Our study and others suggest that the intensity of hyperlactatemia also seems to be associated with the severity of illness and the risk of death. Chen et al. reported that lactate was significantly lower in moderately ill patients, at a median of 1.7 mmol/L (IQR, 1.4–1.9 mmol/L), than in critically ill patients, who had a median of 3.3 mmol/L (IQR, 2.3–4.4 mmol/L) (19). Bruno et al. [2021] conducted a multivariate analysis in a population of 826 patients over 70 years of age with COVID-19 and with baseline lactate ≥2 mmol/L in the ICU. They found that the maximum lactate concentration on day 1 was independently associated with ICU mortality (OR 1.06, 95% CI: 1.02–1.11; P=0.007) (21).
Some studies have shown that lactate >1.5 mmol/L is associated with a higher mortality rate (22,23). A post hoc analysis of 665 patients treated with vasopressin in the Septic Shock trial revealed that patients with lactate levels between 1.4 and 2.3 mmol/L had a significantly higher risk of 28-day death and organ dysfunction than those with a maximum lactate concentration of 1.4 mmol/L (24).
Our study found no association between hyperlactatemia and the need for vasopressors. The hyperlactatemia in these patients can be explained by nonhypoxic mechanisms such as the intense systemic inflammation that they suffered and, particularly in our study population, severe lung injury (14,25). However, hyperlactatemia can occur even without obvious shock in critically ill patients and indicates a worse prognosis (4,26). Although our patients with lactate ≥2 mmol/L used more vasopressors and had lower mean arterial pressure, which may be related to the use of sedation and deep intravenous analgesia, the differential evaluation between hypoxic and nonhypoxic causes of hypoxic disorders was limited by the lack of perfusion variables, our analysis being restricted to the use or nonuse of vasopressors, but vasopressor use is not synonymous with shock.
In 2020, the ICU mortality rates of patients with COVID-19 varied widely between several studies, reaching a maximum of 88% (12,27-29). Our mortality rate was high but similar to that reported in epidemiological studies in Brazil (12) and Latin America (29). A systematic review found an overall ICU mortality rate of 25.7%, suggesting that poor outcomes at the beginning of the pandemic may have been related to resource rationing in overloaded ICUs (30).
Our study was retrospective and single-center in nature. The analysis was limited to the data available in the medical records of the patients. The sample was collected at the beginning of the pandemic in 2020, and the inexperience of health professionals in the care of patients with COVID-19, the resource exhaustion, the emotional impacts, and the lack of vaccines may have influenced the outcomes. A large proportion of the patients admitted to the ICU of the Hospital de Clínicas de Porto Alegre came from other institutions, so their initial care was heterogeneous. Type 2 diabetes was more common among those with hyperlactatemia, but we did not have information on the use of metformin or thiamine deficiency in these patients, which could have contributed to this increase (18). Other factors known to increase serum lactate levels include the use of intravenous lorazepam, associated with propylene glycol-induced lactic acidosis, and antiretroviral therapy, particularly with nucleoside reverse transcriptase inhibitors (NRTIs), in human immunodeficiency virus (HIV)-positive patients, which may lead to mitochondrial dysfunction. However, intravenous lorazepam is not available for use in Brazil, and no patients in our cohort were undergoing antiretroviral therapy. Additionally, the lack of perfusion and serial lactate variables made our interpretation of hyperlactatemia incomplete (31).
For the second and third days, it was not feasible to apply a Poisson regression model with robust variances to assess whether lactate levels were independently associated with increased mortality. This limitation was due to a substantial reduction in the residual sample size, as many patients succumbed after the first day, reflecting the overall high mortality rate in this sample.
Conclusions
Although this is an observational and retrospective study with inherent limitations, such as the absence of dynamic perfusion variables and additional data that could influence the interpretation of hyperlactatemia, the findings indicate that hyperlactatemia, even at low levels (≥1.6 mmol/L) within the first 24 hours of admission, is an independent and significant prognostic marker in critically ill patients with severe COVID-19 admitted to the ICU. These results underscore the importance of early lactate measurement as a crucial tool for identifying high-risk patients and guiding clinical decisions with greater precision.
Acknowledgments
We would like to thank Wagner Azeredo Azevedo and Henrique Wong Jacques for their contribution to data collection.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-116/rc
Data Sharing Statement: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-116/dss
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-116/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-116/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre (Certificate of Presentation and Ethical Assessment No. 51976521.8.0000.5327, with Substantial Opinion No. 5.080.112). A review of the hospital records of patients admitted to the ICU was authorized, with the waiver of free and informed consent. The study adhered to the Declaration of Helsinki (as revised in 2013), and the anonymity of patients was preserved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bakker J, Coffernils M, Leon M, et al. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest 1991;99:956-62. [PubMed]
- Bakker J, Gris P, Coffernils M, et al. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 1996;171:221-6. [Crossref] [PubMed]
- Friedman G, Berlot G, Kahn RJ, et al. Combined measurements of blood lactate concentrations and gastric intramucosal pH in patients with severe sepsis. Crit Care Med 1995;23:1184-93. [Crossref] [PubMed]
- Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit Care 2004;8:R60-5. [Crossref] [PubMed]
- Hernandez G, Bruhn A, Castro R, et al. Persistent Sepsis-Induced Hypotension without Hyperlactatemia: A Distinct Clinical and Physiological Profile within the Spectrum of Septic Shock. Crit Care Res Pract 2012;2012:536852. [Crossref] [PubMed]
- Calvete JO, Schonhorst L, Moura DM, et al. Acid-base disarrangement and gastric intramucosal acidosis predict outcome from major trauma. Rev Assoc Med Bras (1992) 2008;54:116-21. [Crossref] [PubMed]
- Gattinoni L, Vasques F, Camporota L, et al. Understanding Lactatemia in Human Sepsis. Potential Impact for Early Management. Am J Respir Crit Care Med 2019;200:582-9. [Crossref] [PubMed]
- De Backer D. Lactic acidosis. Intensive Care Med 2003;29:699-702. [Crossref] [PubMed]
- Nedel WL, Strogulski NR, Kopczynski A, et al. Association Between Hyperlactatemia, Perfusional Parameters, and Lymphocyte Mitochondrial Dysfunction in Septic Shock Patients. Shock 2022;57:378-83. [Crossref] [PubMed]
- Calvete J, Schonhorst L, Moura DM, et al. The prognostic value of gastric tonometry in severe polytrauma patients. Crit Care 2002;6:175. [Crossref]
- Alves FA, Sant’Anna UL, Oliveira E, et al. The prognostic value of the initial hemodynamic course in patients with circulatory failure. Revista Brasileira de Terapia Intensiva 1998;10:68-75.
- Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med 2021;9:407-18. [Crossref] [PubMed]
- Kowsar R, Rahimi AM, Sroka M, et al. Risk of mortality in COVID-19 patients: a meta- and network analysis. Sci Rep 2023;13:2138. [Crossref] [PubMed]
- Iepsen UW, Plovsing RR, Tjelle K, et al. The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism. Exp Physiol 2022;107:665-73. [Crossref] [PubMed]
- Carpenè G, Onorato D, Nocini R, et al. Blood lactate concentration in COVID-19: a systematic literature review. Clin Chem Lab Med 2021;60:332-7. [Crossref] [PubMed]
- Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005;31:1345-55. [Crossref] [PubMed]
- Wendel Garcia PD, Fumeaux T, Guerci P, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020;25:100449. [Crossref] [PubMed]
- Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One 2020;15:e0241955. [Crossref] [PubMed]
- Chen SL, Feng HY, Xu H, et al. Patterns of Deterioration in Moderate Patients With COVID-19 From Jan 2020 to Mar 2020: A Multi-Center, Retrospective Cohort Study in China. Front Med (Lausanne) 2020;7:567296. [Crossref] [PubMed]
- Vassiliou AG, Jahaj E, Ilias I, et al. Lactate Kinetics Reflect Organ Dysfunction and Are Associated with Adverse Outcomes in Intensive Care Unit Patients with COVID-19 Pneumonia: Preliminary Results from a GREEK Single-Centre Study. Metabolites 2020;10:386. [Crossref] [PubMed]
- Bruno RR, Wernly B, Flaatten H, et al. Lactate is associated with mortality in very old intensive care patients suffering from COVID-19: results from an international observational study of 2860 patients. Ann Intensive Care 2021;11:128. [PubMed]
- Vincent JL, Quintairos E. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016;20:257. [Crossref] [PubMed]
- Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670-7. [Crossref] [PubMed]
- Wacharasint P, Nakada TA, Boyd JH, et al. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock 2012;38:4-10. [PubMed]
- De Backer D, Creteur J, Zhang H, et al. Lactate production by the lungs in acute lung injury. Am J Respir Crit Care Med 1997;156:1099-104. [Crossref] [PubMed]
- Crowl AC, Young JS, Kahler DM, et al. Occult hypoperfusion is associated with increased morbidity in patients undergoing early femur fracture fixation. J Trauma 2000;48:260-7. [Crossref] [PubMed]
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934-43. [PubMed]
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. [Crossref] [PubMed]
- Reyes LF, Bastidas A, Narváez PO, et al. Clinical characteristics, systemic complications, and in-hospital outcomes for patients with COVID-19 in Latin America. LIVEN-Covid-19 study: A prospective, multicenter, multinational, cohort study. PLoS One 2022;17:e0265529. [Crossref] [PubMed]
- Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 2020;24:285. [Crossref] [PubMed]
- Hernandez G, Luengo C, Bruhn A, et al. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care 2014;4:30. [Crossref] [PubMed]
Cite this article as: Petrochi DJ, Moraes RB, Friedman G. Hyperlactatemia in critically ill patients with COVID-19 is associated with increased mortality. J Emerg Crit Care Med 2025;9:9.


