
Evaluation of patient outcomes associated with lactated ringers versus normal saline in patients presenting with diabetic ketoacidosis in a multicenter health system
Introduction
Diabetic ketoacidosis (DKA) accounts for a significant healthcare burden, leading to hundreds of thousands of hospital days each year in the United States, affecting both children and adults (1-3). DKA is characterized by a triad of hyperglycemia, metabolic acidosis, and ketonemia (2,4). Treatment for this emergency includes insulin therapy, electrolyte supplementation, and fluid administration (2,4). Fluid resuscitation is essential for the treatment of DKA as dehydration may result in a fluid deficit that is 15% of total body weight (4). Administering fluids can decrease counter-regulatory hormones, improve renal function, assist in reversing shock caused by volume depletion, increase renal glucose clearance, and enhance insulin sensitivity (5). According to the American Diabetes Association (ADA) clinical practice guidelines, normal saline (NS) is recommended as the fluid of choice for patients with DKA (2). However, recent evidence suggests that lactated ringers (LR) may offer significant benefits in this patient population (6-10).
NS is a cost-effective, hypertonic, mildly acidic fluid (11). Despite its widespread use, NS poses risks such as hyperchloremic acidosis and acute kidney injury due to its high chloride (Cl) content (8,10,12). Resuscitating patients with intravenous fluids that contain high Cl content may lead to decreased renal blood flow which may contribute to dehydration-related acute kidney injury (AKI) (1,12,13). The concentrations of sodium, potassium, Cl, calcium, and lactate vary between human plasma, LR, and NS (Table 1) (11). However, LR more closely mimics the electrolyte composition of human plasma, potentially reducing these risks and providing an alkalizing effect through lactate metabolism (11,14,15). With any intravenous fluid resuscitation therapy, precautions should be taken in patients with cardiac, renal, or hepatic impairment due to the risk of volume overload (14-16).
Table 1
| Electrolyte | Human plasma, range | LR | NS |
|---|---|---|---|
| Sodium (mEq/L) | 135–145 | 130 | 154 |
| Potassium (mEq/L) | 3.1–5 | 4 | 0 |
| Chloride (mEq/L) | 94–111 | 109 | 154 |
| Calcium (mEq/L) | 2.2–2.6 | 2.7 | 0 |
| Lactate (mEq/L) | 1–2 | 28 | 0 |
LR, lactated ringers; NS, normal saline.
Previous randomized clinical trials comparing the use of balanced crystalloids, such as LR vs. NS in other patient populations, have demonstrated the benefits of LR (13,17,18). Balanced crystalloids for fluid management in critically ill adults resulted in lower rates of death and major adverse kidney events (17). Other studies evaluating patients presenting with DKA revealed a reduced time to resolution in those receiving balanced crystalloids compared to NS (6,7,9,13). Due to an increasing amount of favorable data for the use of LR, the health-system updated the standardized institutional DKA order set to implement LR as the new fluid therapy of choice. The purpose of this study is to assess the impacts of intravenous fluid selection on patient outcomes in DKA in a multicenter health-system. We present this article in accordance with the STROBE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-101/rc).
Methods
Patients
This multicenter, observational, retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and expedited by the institutional review board at the University of Texas Health Science Center in Houston, TX and declared exempt from human subjects research. Individual consent for this retrospective analysis was waived. The study consisted of four community hospitals within the same health-system. Adults aged ≥18 years old who presented to any of the four community emergency departments receiving orders via the DKA order set were included. The LR cohort was evaluated from January 2021 to August 2021 and the NS cohort was evaluated from January 2020 to August 2020. The diagnosis of DKA was consistent with ADA clinical practice guidelines: arterial pH ≤7.3, anion gap (AG) >10 mEq/L, serum sodium bicarbonate ≤18 mEq/L, serum blood glucose >250 mg/dL, and osmolality <320 mOsm/kg. AG was manually calculated by subtracting potassium from AG values reported on the electronic medical record to determine resolution of DKA per ADA guidelines. Patients were excluded if they presented with hyperchloremia on admission (serum Cl >110 mEq/L), osmolality >320 mOsm/kg, or if they received >1 L of NS in the LR cohort or >1 L of LR in NS cohort. Patient demographics, past medical history, and potassium supplementation information were also collected.
Study outcomes
The primary outcome was the time to resolution of DKA according to ADA clinical practice guidelines including a serum blood glucose <200 mg/dL and two of the following criteria: serum bicarbonate ≥15 mEq/L, venous pH >7.3, or AG ≤12 mEq/L (3). Time to resolution was calculated by subtracting the date and time of first lab results meeting the defined criteria from the date and time of the first lab results meeting the defined criteria of DKA resolution. Secondary outcomes included time to discontinuation of insulin infusion, total amount of fluid received in L, incidence of hyperchloremia (serum Cl >110 mEq/L), and amount of potassium supplementation required. Secondary outcomes were collected in accordance with first laboratory results consistent with DKA to time of resolution. Outcomes were further analyzed by site. A subgroup analysis examined these outcomes stratified by comorbidities that could be affected by fluid administration, including congestive heart failure (CHF), chronic kidney disease (CKD), and cirrhosis.
Statistical analysis
Demographic data was analyzed via descriptive statistics and chi-square analysis. Statistical analysis was performed via R Studio 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). A Shapiro-Wilk analysis was performed to determine the parametric nature of continuous variables. Non-parametric, continuous variables were reported as median values and interquartile ranges (IQRs) and analyzed using the Mann-Whitney U test. Parametric continuous values were analyzed using t-tests. Categorical variables were analyzed via Chi-squared analysis. For statistical interpretations, a P value <0.05 was considered statistically significant. A post-hoc calculation was performed to assess study power.
Results
A total of 1,085 patients were screened for eligibility and 723 (66.6%) were excluded (Figure 1). This study included 362 (33.3%) patients, of whom 136 (12.5%) were in the LR cohort and 226 (20.8%) were in the NS cohort. Baseline demographic data are summarized in Table 2. Age was significantly different among the cohorts with a median age of 43.5 vs. 47.4 years in the LR cohort and NS cohort, respectively (P=0.044). There were also significantly more males in the NS cohort [120 (62.8%) vs. 71 (37.2%), P=0.048]. Otherwise, the two cohorts were well balanced and there were no significant differences in race or weight among the two cohorts. There was a significant reduction in the median time to DKA resolution in the LR cohort (14.4 hours; IQR, 11.2–20.7 hours) when compared to the NS cohort (17.2 hours; IQR, 11.5–25.9 hours) (P=0.02) (Figure 2A). However, when analyzed by hospital site, there was no significant difference in time to DKA resolution amongst the two fluid cohorts (Figure 2B).
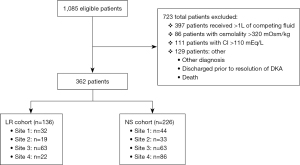
Table 2
| Demographics | LR (n=136) | NS (n=226) | P value |
|---|---|---|---|
| Age (years) | 43.5 (29.5–56) | 47.4 (31–61) | 0.044 |
| Male | 71 (37.2) | 120 (62.8) | 0.048 |
| Race | 0.70 | ||
| Caucasian | 32 (36.8) | 55 (63.2) | |
| African American | 29 (37.2) | 49 (62.8) | |
| Asian | 2 (66.6) | 1 (33.3) | |
| Hispanic | 2 (66.6) | 1 (33.3) | |
| Other | 70 (37.6) | 116 (62.4) | |
| Weight (kg) | 77 (61.6–90.5) | 78 (62.5–87.8) | 0.67 |
| Past medical history | |||
| Diabetes | 112 (37.2) | 189 (62.8) | 0.75 |
| CKD | 10 (28.6) | 25 (71.4) | 0.25 |
| Cirrhosis | 2 (100.0) | 0 (0.0) | N/A |
| CHF | 1 (16.7) | 5 (83.3) | 0.29 |
| Other (acute coronary syndrome, thyroid disease, hypertension, etc.) | 64 (29.9) | 150 (70.0) | <0.001 |
| None | 14 (51.9) | 13 (48.1) | 0.11 |
Data are presented as median (IQR) or n (%). LR, lactated ringers; NS, normal saline; CKD, chronic kidney disease; CHF, congestive heart failure; IQR, interquartile range.

The median Cl level in both treatment cohorts was 104 mEq/L until time to DKA resolution (P=0.61) and there was no significant difference in patient incidence of hyperchloremia until time to resolution as shown in Figure 3 [10 (7.4%) vs. 12 (5.3%), P=0.42]. There was a nonsignificant shorter median time to discontinuation of the insulin infusion in the LR cohort (21.4 hours; IQR, 16.5–31.1 hours) compared to the NS cohort (24.2 hours; IQR, 17.2–35.5 hours) (P=0.18). When analyzed by site, there was a significant reduction in time to discontinuation of insulin infusion in the LR cohort (19.7 hours; IQR, 15.1–26.4 hours) vs. the NS cohort (24.2 hours; IQR, 17.7–35.5 hours) for site 3 (P=0.048). The NS cohort received a nonsignificant lower median volume of fluid and required less median potassium supplementation than the LR cohort (Table 3).
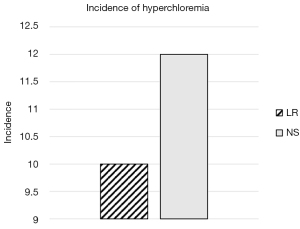
Table 3
| Outcome | Overall | Site 1 | Site 2 | Site 3 | Site 4 |
|---|---|---|---|---|---|
| Sample size | |||||
| LR | 136 (37.6) | 32 (42.1) | 19 (36.5) | 63 (50.0) | 22 (20.4) |
| NS | 226 (62.4) | 44 (57.9) | 33 (63.5) | 63 (50.0) | 86 (79.6) |
| Amount of fluid received (L) | |||||
| LR | 6 (4–7.3) | 6 (3.3–7.8) | 4 (3.1–8) | 6.5 (4.5–8) | 5 (3–6) |
| NS | 5.2 (4–7) | 5.2 (3.6–6.9) | 5 (4–6) | 6 (3–7) | 6 (4–7) |
| Time to discontinuation of insulin infusion (h) | |||||
| LR | 22.7 (16.5–31.1) | 27.1 (18.3–39.1) | 25.7 (12.5–31.9) | 19.7 (15.1–26.4)* | 22.5 (18.8–34.3) |
| NS | 24.2 (17.2–35.5) | 20.6 (10.8–37.9) | 23.3 (17–30.7) | 24.7 (17.7–35.5)* | 24.7 (19.6–36.3) |
| Amount of potassium supplementation required (mEq) | |||||
| LR | 60 (15–114.5) | 52 (0–117.5) | 50 (0–80) | 64 (30–143) | 52 (10–100) |
| NS | 44 (10–97.5) | 35 (0–77.5) | 40 (0–67) | 60 (40–123.3) | 40 (0–85.5) |
| ICU LOS (days) | |||||
| LR | 1.5 (0.8–2.6)** | 1.9 (1.1–2.7) | 1.6 (0.9–3.5) | 1.6 (1–3.5) | 0 (0–2.2) |
| NS | 1.1 (0–1.8)** | 1.7 (1–2) | 1.2 (1–1.8) | 1.3 (0.9–3.9) | 0 (0–0) |
| Overall LOS (days) | |||||
| LR | 3.2 (2.3–6.4) | 3.2 (2.1–4.1) | 3 (2.55–2.63)*** | 4.4 (2.4–14.3) | 2.4 (1.8–5.3) |
| NS | 3.3 (2.2–6.1) | 3.5 (2.2–6.4) | 2.2 (1.9–3.3)*** | 3.9 (2.5–8.1) | 3.8 (2.4–6.2) |
Data are presented as n (%) or median (IQR). Findings are presented for overall cohorts and also shown by site. *, indicates significance between cohorts, P=0.049; **, indicates significance between cohorts, P<0.001; ***, indicates significance between cohorts, P<0.01. LR, lactated ringers; NS, normal saline; ICU, intensive care unit; LOS, length of stay; IQR, interquartile range.
When assessing length of stay (LOS), there was a significantly longer intensive care unit (ICU) LOS in the LR cohort compared to the NS cohort [1.52 (IQR, 0.81–2.61) vs. 1.07 (IQR, 0–1.83) days; P<0.001] (Table 3). There was no significant difference in overall LOS between the two cohorts (P=0.68) or when analyzed by hospital site. Results of the subgroup analysis on patients with CKD, cirrhosis, and CHF are listed in Table 4. None of the outcomes of the subgroup analysis, including time to resolution, amount of fluid received, ICU LOS, or overall LOS, were found to be significant between the two fluid cohorts.
Table 4
| Comorbidity | Number of receiving LR & NS | Time to resolution of DKA (h) | Amount of fluid received (L) | ICU LOS (days) | Overall LOS (days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | NS | LR | NS | LR | NS | LR | NS | LR | NS | |||||
| Chronic kidney disease (n=35) | 25 (71.4) | 10 (28.6) | 17.8 (12.5–28.6) | 23.9 (11.8–60.0) | 3 (2–3.5) | 3 (2–5) | 2.3 (1–16) | 1.1 (0–2.1) | 11.5 (2.2–18.4) | 4.4 (2.8–9.5) | ||||
| Cirrhosis (n=2) | 2 (100.0) | 0 (0.0) | 20.3 (18.9–21.7) | – | 4 (1.1–7) | – | 12.6 (8.8–16.3) | – | 12.8 (9.0–16.9) | – | ||||
| Congestive heart failure (n=6) | 1 (16.7) | 5 (83.3) | 12 (NA) | 19.1 (12.7–38.4) | 3.5 (NA) | 2.5 (1.5–6) | – | 1.4 (1.3–16.1) | 1.9 (NA) | 6.5 (2.3–18.2) | ||||
Data are presented as n (%) or median (IQR). There were no statistically significant findings when results were analyzed by comorbidities. LR, lactated ringers; NS, normal saline; DKA, diabetic ketoacidosis; IQR, interquartile range; ICU, intensive care unit; LOS, length of stay; NA, not applicable.
Discussion
In this multicenter, observational, retrospective, study comparing patient outcomes associated with the receipt of LR vs. NS in the treatment of DKA, 362 patients were evaluated making it the largest retrospective study to date in this patient population. Demographics were distributed equally among each cohort with the exception of age and gender. Although, these differences are not likely to be clinically significant. Multiple studies have demonstrated a reduction of time to resolution of DKA when utilizing balanced crystalloids in comparison to NS (6,7,9,13,15,18,19). While prior studies have explored the use of LR in managing DKA, this study broadens the scope by evaluating outcomes from patients presenting to multiple community hospital emergency departments. This approach offers greater generalizability compared to studies focused solely on academic settings. For the primary outcome, there was a significant reduction for time to DKA resolution in the LR cohort. When the primary outcome was analyzed by site, a difference was not detected. The lack of significant findings when analyzed by site could be due to the low and varying sample sizes among individual hospital sites and cohorts. All four sites were community hospitals in the same health-system with facility sizes ranging from 81 to 542 beds and with various patient acuity levels. This allowed for enhanced population generalizability.
It was hypothesized that LR would have a lower incidence of hyperchloremia due to the difference in Cl content between the two fluids (11,15). A meta-analysis demonstrated that fluids with high Cl content did not affect mortality but were associated with a significantly higher risk of acute kidney injury and hyperchloremic/metabolic acidosis (20). Therefore, this study excluded patients presenting with serum Cl concentration >110 mEq/L in an effort to detect incidence of hyperchloremia during treatment. Ultimately, this analysis was not able to replicate previous trial results demonstrating higher incidences of hyperchloremia in those treated with NS. This could be due the exclusion of patients of hyperchloremia at presentation and low number of patients who have a history of CKD in this sample size. Patients with CKD are more likely to develop hyperchloremia when receiving fluid resuscitation, particularly when receiving Cl-rich fluids like NS. The impaired ability of the kidneys to handle an excess Cl load in patients with CKD makes them theoretically more susceptible to hyperchloremic metabolic acidosis during fluid resuscitation (21).
This study’s findings align with prior research demonstrating the benefits of balanced crystalloids in critically ill patients and patients with DKA (6,7,9,13). Based on these results, LR may be more beneficial in those with renal dysfunction or acidosis on admission. Furthermore, quantities of potassium supplementation received was analyzed due to controversy regarding LR use in acidotic patients due to the potassium content in the fluid. Although not statistically significant, the findings of this study demonstrated those receiving LR required more potassium supplementation than patients in the NS cohort (P=0.10). This has been explored in previous studies with mixed results (15,21,22). Although LR contains minimal amounts of potassium (4 mEq/L) while NS does not, the effects on potassium supplementation requirements vary (14). Given the more acidic effects of NS, potassium may shift extracellularly at higher degrees, mask the true potassium deficit, and result in less potassium supplementation requirements in the NS cohort (21,23). Alternatively, balanced crystalloids, such as LR, have a mild alkalinizing effect (14). This can help reduce extracellular potassium levels by promoting intracellular potassium shifts and requiring more potassium supplementation (21,23). This could be due to the potential acidosis related to NS administration causing extracellular shifting of potassium (15,16). Conversely, the lactate content in LR is predominantly metabolized to bicarbonate which may assist in intracellular potassium shifts resulting in less extracellular potassium requirements (14).
The lack of significant findings in secondary outcomes, such as amount of fluid administration and time to discontinuation of insulin, may be due to differences in practice at the various hospital sites. At some institutions included in this analysis, patients may have received 0.45% NS during various stages of resuscitation. This may have resulted in a falsely lower report of saline-containing fluids administered to patients in the NS group as reflected in Table 3. Additionally, the timing and process of discontinuing an insulin infusion and transferring a patient out of the ICU can differ based on policy and protocols of different institutions. For example at one of the included institutions, transition of insulin infusions to basal-bolus insulin regimens do not commonly occur outside of morning interdisciplinary rounds. This could add to less variation among the cohorts with regard to insulin infusion discontinuation, ICU LOS, and overall LOS. Patients might have also remained in the ICU despite resolution of DKA due to unrelated complications and other comorbidities. The subgroup analysis aimed to provide additional information regarding common comorbidities that may be affected by excess fluid resuscitation. However, there were no significantly different findings among the cohorts. This finding aligns with a recently published trial, which demonstrated that intravenous fluid restriction in patients with septic shock did not reduce mortality compared to standard fluid therapy (24). Additional prospective trials should examine the differences between LR and NS regarding these comorbidities among patients with DKA.
This analysis concluded that median time to DKA resolution was reduced in patients who received LR. Previous studies have found the benefit of using fluids other than NS in the treatment of DKA, but many of these trials did not meet the predefined power analysis. A post-hoc power analysis was calculated to confirm the sample size was large enough to detect significant differences in this study. The post-hoc power analysis revealed a statistical power of 85.2% as calculated with α =0.05 revealing that the sample size was adequate in this study. This study sample was larger than previous trials despite its strict inclusion and exclusion criteria. In this study, only patients who reached resolution of DKA were included and those who were discharged prior to resolution per ADA guidelines or were deceased were excluded (1). Additionally, this study is a multicenter study that examined incidence of hyperchloremia until time to resolution and the effect of fluids for the treatment of DKA in comorbidities such as CKD, CHF, and cirrhosis. Another unique aspect was excluding those with Cl levels >110 mEq/L on admission and those who received greater than 1 L of fluid of the other cohort reduced confounding factors. The results of this study apply specifically to DKA patients treated with LR.
Limitations
Strengths of this study include its large sample size and multicenter design, enhancing the generalizability of the findings. However, this study has several limitations. First, this study was retrospective and data was limited to the sample size available. When selecting our study cohort, patients who received greater than 1 L of fluid from the other treatment group were excluded to minimize bias. Unfortunately, this evaluation was not able to quantify the amount of undocumented fluids provided by emergency medical services among patients presenting via ambulance. Patients with severe DKA might have been started on other infusions whose fluid volume was not accounted for in the medication review as well. Additionally, AG was manually calculated in this study by subtracting potassium from AG values to determine resolution of DKA per ADA guidelines whose calculation of AG does not include potassium levels. Therefore, treatment decisions may have been impacted due to the difference in AG calculations.
Conclusions
Currently, the ADA guideline recommendations support NS as the fluid of choice for treatment of DKA. This study provides evidence that LR may be preferred over NS for treating DKA, reducing the time to resolution. There was no difference in secondary outcomes including: volume of fluid received, time to discontinuation of insulin infusion, amount of potassium supplementation, and overall length of stay. Further research, particularly prospective randomized controlled trials, is needed to confirm these findings and assess their broader applicability. These findings have the potential to inform future protocols across healthcare systems, ultimately contributing to the creation of healthier communities, now and for generations to come.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-101/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-101/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This multicenter, observational, retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and expedited by the institutional review board at the University of Texas Health Science Center in Houston, TX and declared exempt from human subjects research and was considered quality improvement. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335-43. [Crossref] [PubMed]
- Gosmanov AR, Gosmanova EO, Kitabchi AE. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. In: Feingold KR, Anawalt B, Blackman MR, et al. editors. Endotext. South Dartmouth, MA, USA: MDText.com, Inc.; 2021 [cited 2024 Jul 15]. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279052/
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician 2013;87:337-46. [PubMed]
- Lizzo JM, Goyal A, Gupta V. Adult Diabetic Ketoacidosis. Treasure Island, FL, USA: StatPearls Publishing; 2024 [cited 2024 Jul 15]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560723/
- Jayashree M, Williams V, Iyer R. Fluid Therapy For Pediatric Patients With Diabetic Ketoacidosis: Current Perspectives. Diabetes Metab Syndr Obes 2019;12:2355-61. [Crossref] [PubMed]
- Jamison A, Mohamed A, Chedester C, et al. Lactated Ringer's versus normal saline in the management of acute diabetic ketoacidosis (RINSE-DKA). Pharmacotherapy 2024;44:623-30. [Crossref] [PubMed]
- Johnson J, Drincic A, Buddenhagen E, et al. Evaluation of a Protocol Change Promoting Lactated Ringers Over Normal Saline in the Treatment of Diabetic Ketoacidosis. J Diabetes Sci Technol 2024;18:549-55. [Crossref] [PubMed]
- Liu Y, Zhang J, Xu X, et al. Comparison of balanced crystalloids versus normal saline in patients with diabetic ketoacidosis: a meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2024;15:1367916. [Crossref] [PubMed]
- Carrillo AR, Elwood K, Werth C, et al. Balanced Crystalloid Versus Normal Saline as Resuscitative Fluid in Diabetic Ketoacidosis. Ann Pharmacother 2022;56:998-1006. [Crossref] [PubMed]
- Alghamdi NA, Major P, Chaudhuri D, et al. Saline Compared to Balanced Crystalloid in Patients With Diabetic Ketoacidosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Explor 2022;4:e0613. [Crossref] [PubMed]
- Noritomi DT, Pereira AJ, Bugano DD, et al. Impact of Plasma-Lyte pH 7.4 on acid-base status and hemodynamics in a model of controlled hemorrhagic shock. Clinics (Sao Paulo) 2011;66:1969-74. [Crossref] [PubMed]
- Zhou FH, Liu C, Mao Z, et al. Normal saline for intravenous fluid therapy in critically ill patients. Chin J Traumatol 2018;21:11-5. [Crossref] [PubMed]
- Self WH, Evans CS, Jenkins CA, et al. Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials. JAMA Netw Open 2020;3:e2024596. [Crossref] [PubMed]
- Singh S, Kerndt CC, Davis D. Ringer's Lactate. Treasure Island, FL, USA: StatPearls Publishing; 2024 [cited 2024 Jul 15]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK500033/
- Mahler SA, Conrad SA, Wang H, et al. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med 2011;29:670-4. [Crossref] [PubMed]
- Nagami GT. Hyperchloremia - Why and how. Nefrologia 2016;36:347-53. [Crossref] [PubMed]
- Semler MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 2018;378:829-39. [Crossref] [PubMed]
- Ramanan M, Attokaran A, Murray L, et al. Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): a cluster, crossover, randomized, controlled trial. Intensive Care Med 2021;47:1248-57. [PubMed]
- Zampieri FG, Machado FR, Biondi RS, et al. Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021; Epub ahead of print. [Crossref] [PubMed]
- Hammond NE, Zampieri FG, Di Tanna GL, et al. Balanced Crystalloids versus Saline in Critically Ill Adults - A Systematic Review with Meta-Analysis. NEJM Evid 2022;1:EVIDoa2100010.
- Song K, Yang T, Gao W. Association of hyperchloremia with all-cause mortality in patients admitted to the surgical intensive care unit: a retrospective cohort study. BMC Anesthesiol 2022;22:14. [Crossref] [PubMed]
- Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer's lactate versus normal saline: a randomized controlled trial. QJM 2012;105:337-43. [Crossref] [PubMed]
- Honore PM, Mugisha A, Kugener L, et al. The causal link between hyperchloremia and acute kidney injury is yet to be conclusively established: we are not sure. Crit Care 2020;24:271. [Crossref] [PubMed]
- Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N Engl J Med 2022;386:2459-70. [Crossref] [PubMed]
Cite this article as: Castillo RM, Madhavaram J, Tran M, Chilappa R, Mercer KJ. Evaluation of patient outcomes associated with lactated ringers versus normal saline in patients presenting with diabetic ketoacidosis in a multicenter health system. J Emerg Crit Care Med 2025;9:11.


