Efficacy and safety of intravenous oxycodone for general anesthesia in children
Introduction
Oxycodone is a kind of semisynthetic opioid that has analgesic effect for moderate to severe pain. Unlike morphine, which is an agonist of µ-opioid receptors, oxycodone acts on k-opioid receptor (1). Furthermore, oxycodone is metabolized in the liver to oxymorphone. The later metabolite is a more potent opioid agonist with higher binding affinity to µ-opioid receptors compared to oxycodone. However, subsequent researches revealed that the action of oxycodone is far more complex that its effect is mediated by different receptors in different situations (2,3). Oxycodone can be administered orally and intravenously. The bioavailability of oral administration of oxycodone averages 60–87% (4). Oxycodone is eliminated from sweat and urine and thus it accumulates in patients with renal dysfunction.
Since its first synthesis 100 years ago, oxycodone has been widely used in clinical practice (4). For example, oral formulas of oxycodone has been widely used for cancer pain (5-9). Some other studies have reported the efficacy and safety of oxycodone for the management of postoperative pain [e.g., in the form of patient controlled analgesia (PCA)] (10-12). These studies consistently show that oxycodone is a safe and effective analgesic. There is also evidence supporting that IV oxycodone may be associated with greater pain control, fewer severe adverse events, and faster onset of action, although the results are not consistent across all studies (13-16). Oxycodone used for intraoperative analgesia has also been reported in adult but the evidence is scarce (17,18). In pediatric patients and infants, oxycodone administered postoperatively has been reported (19-21). However, to the best of our knowledge, there is no study reporting on intravenous use of oxycodone for analgesia (administered before surgery) in children. In the present study, we aimed to explore the efficacy and safety of analgesia with intravenous oxycodone for children.
Methods
Study population
The study was a retrospective study enrolling children (<15 years old) underwent general anesthesia from May 2015 to November 2015. Medical chart of eligible patients were reviewed. Patients were eligible if they fulfill the following criteria: (I) age younger than 15 years old; (II) patients underwent general anesthesia. Patients would be excluded if they (I) had on analgesics for chronic pain before operation; (II) underwent local anesthesia and/or spinal anesthesia; (III) used fentanyl for anesthesia; and (IV) were neonates (<28 days) or infants (<12 months). This was a comparative study by using historical controls. The study was approved by the institutional review board of Jinhua Municipal Central Hospital. Informed consent was waived due to retrospective nature of the study.
Anesthesia procedure
Our institution started to use intravenous oxycodone for anesthesia for pediatric patients from October 2015. In the study group, anesthesia was induced by propofol (3 mg/kg), cisatracurium (0.1–0.15 mg/kg) and oxycodone (0.2–0.4 mg/kg). In the control group, the oxycodone was replaced by sulfentanil (0.3–0.6 ug/kg). Both groups had remifentanil (11–13 ug/kg/hr) administered continuously via intravenous pump during operation. Anesthesia was maintained by propofol (6–9 mg/kg/hr) and cisatracurium (0.05–0.1 mg/kg/hr) in all patients. After operation, remifentanil was discontinued and patients were sent to the post-anesthesia care unit (PACU) for recovery. Volume controlled mechanical ventilation was instituted to reach a tidal volume of 10–12 mL/kg, and respiratory rate was set to maintain an end-tidal carbon dioxide level of 36–44 mmHg. During anesthesia period, oxygen saturation, heart rate, respiratory rate, blood pressure and end-tidal carbon dioxide were monitored continuously and recorded every 5 minutes. According to our local anesthesia protocol, patients were weaned from mechanical ventilation and extubated once they were awaken, hemodynamically stable and recovered stable respiration (tidal volume >6 mL/kg persistently).
Postoperative follow up
Postoperative pain was measured by using visual analogue scale (VAS) score if possible (22). VAS score ranged from 0 to 10 points, with 0 represented no pain and 10 points represented the worst the patient had ever experienced. Postoperative analgesics required were also recorded, which included diclofenac, tramadol, parecoxib, tetrandrine, flurbiprofen and somedon. Patients were followed for 72 hours postoperatively. The postoperative adverse events such as nausea, vomiting and itching and dizziness were also recorded within 72 hours. These adverse events were coded as dichotomous variables with “yes” or “no” status.
Statistical analysis
Continuous variable such as age and body weight were expressed as mean (standard deviation) if they were normally distributed. Otherwise, they were expressed as median (interquartile range). Univariate analysis was performed to compare the difference of baseline characteristics between oxycodone group and conventional group. Student t-test was employed for data with normal distribution, and Mann-Whitney U test was used for skewed data. Categorical variables such as sex, type of surgery and adverse events were expressed as the number and percentage. Their difference between oxycodone group and conventional group were tested by using Chi-square test.
If there are statistically significant differences in baseline characteristics between oxycodone and control group, analysis would be performed by using propensity score matching. Because missing data were present in the retrospective study and propensity score matching cannot be performed with missing values, we employed multiple imputation (MI) technique to estimate the missing values (23). This method had advantage that it takes account of the uncertainty in missing value estimation. Specifically, we created five complete datasets from original dataset containing missing values. Predictive mean matching was used to predict continuous missing values and logistic regression was used for dichotomous missing values (24). Thereafter, multivariable regression model was performed to examine the association between oxycodone use and postoperative analgesics use, by adjusting for other confounding factors. From clinical perspective, these confounding factors included age, type of surgery, length of operation, and sex. Five models were fitted, from which five coefficients were obtained from each imputed datasets. Finally, these datasets were combined to obtain final results. In this way, the variance would not be underestimated as compared to single imputation (25).
All statistical analyses were performed by using R software (R version 3.2.2), statistical significance was considered at P<0.05.
Results
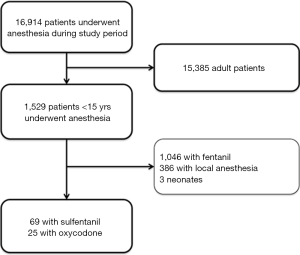
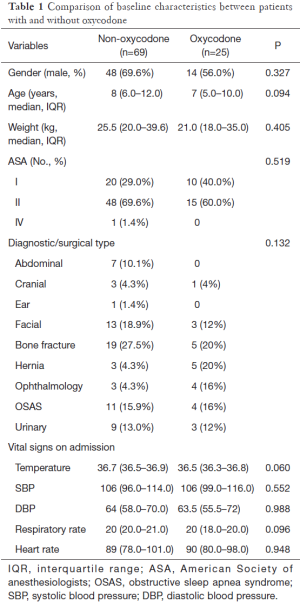
During the study period, a total of 16,914 patients underwent anesthesia in our department (Figure 1). Of them, there were 1,529 children. After exclusion of 1,046 children with fentanyl for anesthesia, 386 children with local anesthesia, and 3 neonates, the remaining 94 children fulfilled our inclusion criteria and were used for analysis. There were 69 children in the control group and 25 children in the oxycodone group (Table 1). There were no significant differences in percentage of male (69.6% vs. 56.0%; P=0.327) and body weight (median: 25.5 vs. 21 kg; P=0.405) between the two groups. Patients in control group appeared to be older than oxycodone group (median: 8 vs. 7 years old; P=0.094). Most children had ASA score of I or II and there was no significant difference between the two groups. However, there was one child with IV ASA score in the control group. There were seven cases of abdominal surgery in the control group, but there is no one in the oxycodone group. However, the statistical significance of the difference in diagnosis/surgical type was not reached. Vital signs on admission were not significantly different between the two groups. However, the control group appeared to have higher temperature [36.7 (36.5–36.9) vs. 36.5 (36.3–36.8); P=0.06] and respiratory rate {20 [20–21] vs. 20 [18–20]; P=0.096} than the oxycodone group.
Full table
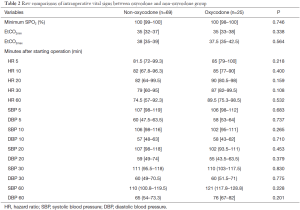
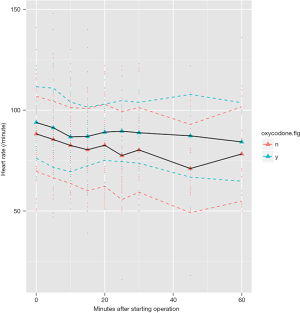
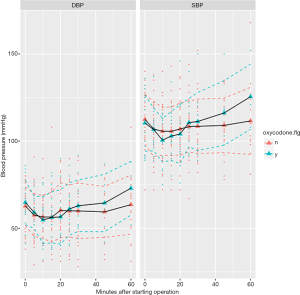
Because we failed to identify covariates that were significantly associated with oxycodone use, propensity score matching was not adopted. During operation we monitored blood pressure and heart rate consecutively and found there was no difference between oxycodone and control group at 5, 10, 20, 30, 60 minutes after starting operation (Table 2). The SPO2 and EtCO2 were both maintained at a safe range. Figure 2 shows the trends of heart rate after starting operation, stratified by oxycodone and control groups. The mean heart rate was slightly higher in the oxycodone group over the entire operation course. However, the standard deviation (dashed line, colors indicate different groups) overlapped with each other. Figure 3 shows the trends of SBP and DBP over the course of operation. There was no significant difference between oxycodone and control groups.
Full table
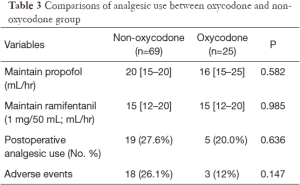
The efficacy of oxycodone could be assessed by the intraoperative use of analgesics and propofol, as well as the postoperative analgesic requirement (Table 3). The propofol {20 [15–20] vs. 16 [15–25] mL/hr; P=0.582} and ramifentanil {15 [12–20] vs. 15 [12–20] mL/hr; P=0.985} requirements were similar between both groups. Postoperative analgesic uses were slightly higher in the control group than oxycodone group (27.6% vs. 20.0%; P=0.636). The incidence of adverse events including nausea, vomiting tended to be higher in the control group (26.1% vs. 12%; P=0.147).
Full table
Discussion
The pilot study showed that oxycodone was able to provide potent analgesic effects during and after operation, and the effect was comparable to the sulfentanil. This is the first study reporting the use of oxycodone for general anesthesia, which provided an alternative analgesic for pediatric anesthesia. However, the study is limited by its retrospective design and small sample size. The promising results of the study provided rationale for designing a randomized controlled trial to explore whether oxycodone can provide additional benefits in pediatric patients undergoing general anesthesia.
In a recent small study, Wang and coworkers compared preemptive analgesic effect of oxycodone and control (normal saline) and found that oxycodone significantly reduced intraoperative hazard ratio (HR) and mean arterial pressure (MAP), and postoperative VAS scores (17). Postoperative tramadol requirement was also lower in the oxycodone group. The message from this study is that oxycodone can provide analgesic effect, but it was not compared to other more commonly used drugs such as sulfentanil. Our study compared oxycodone with sulfentanil as the major analgesics and found that the analgesic effects were similar. When oxycodone was given immediately before the end of surgery or as PCA, it could provide more potent analgesic effect than morphine for visceral pain (10,13,16). Other study showed similar analgesic effect between oxycodone and morphine (15). However, there is evidence that postoperative use of oxycodone is associated with more adverse events (14). This is in contrast to the present results, which suggested lower rate of adverse events in children receiving oxycodone.
The above-mentioned studies reporting analgesic effect of oxycodone were all performed in adult population, and oxycodone was given primarily after surgery for postoperative pain control. There is no report on the intravenous use of oxycodone for general anesthesia in children, partly because pharmacokinetics of oxycodone varied substantially in young children (21). Therefore, there is no data on the efficacy and safety of oxycodone for pediatric anesthesia. There are studies reported on postoperative oxycodone use for pediatric patients. One study involving 18 children showed that oxycodone appeared to cause greater ventilator depression (19). The greatest concentration of oxycodone appeared at 8 minutes after bolus injection. The administration way of oxycodone was similar to our study. The greatest respiratory depression occurred at 8 minutes after injection, during which our children were under general anesthesia and the respiration was controlled by mechanical ventilation. That was why respiratory side effect of oxycodone was not observed in our study. Other study showed that IV oxycodone administered postoperatively could provide supplementary analgesic effect for children (20).
Several limitations of the study need to be mentioned. First, the study was retrospective in nature and there could be selection bias. However, all available baseline demographic and clinical variables were comparable between oxycodone and control groups. At the design stage, we intended to use propensity score matching to exclude confounders. Second, the study is small in sample size and is prone to random errors. Similarly, the statistical power is also limited. Postoperative analgesic use and adverse events tended to be lower in the oxycodone group, but statistical significance was not reached. If statistical power is responsible for this insignificance, further studies with large sample size are mandatory. Third, patients included in our study are heterogeneous. There is evidence that oxycodone may have better analgesic effect for visceral pain. Therefore, further studies restricted to certain types of surgery can have greater statistical power to detect biological efficacy of oxycodone.
In conclusion, the study reported for the first time intravenous use of oxycodone for general anesthesia in pediatric patients. The results showed that oxycodone had comparable analgesic effect to sulfentanil, and tended to have lower postoperative analgesic requirement and less adverse events. Randomized controlled trials are mandatory to confirm the efficacy and safety of oxycodone in pediatric patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Jinhua Municipal Central Hospital on October 10th, 2015 (approval code: 2015-041). Informed consent was waived due to retrospective nature of the study.
References
- Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain 1997;73:151-7. [Crossref] [PubMed]
- Nozaki C, Saitoh A, Kamei J. Characterization of the antinociceptive effects of oxycodone in diabetic mice. Eur J Pharmacol 2006;535:145-51. [Crossref] [PubMed]
- Nozaki C, Kamei J. Involvement of mu1-opioid receptor on oxycodone-induced antinociception in diabetic mice. Eur J Pharmacol 2007;560:160-2. [Crossref] [PubMed]
- Leppert W. Role of oxycodone and oxycodone/naloxone in cancer pain management. Pharmacol Rep 2010;62:578-91. [Crossref] [PubMed]
- Davis M, Goforth HW, Gamier P. Oxycodone combined with opioid receptor antagonists: efficacy and safety. Expert Opin Drug Saf 2013;12:389-402. [Crossref] [PubMed]
- Oosten AW, Oldenmenger WH, Mathijssen RH, et al. A Systematic Review of Prospective Studies Reporting Adverse Events of Commonly Used Opioids for Cancer-Related Pain: A Call for the Use of Standardized Outcome Measures. J Pain 2015;16:935-46. [Crossref] [PubMed]
- Schmidt-Hansen M, Bennett MI, Arnold S, et al. Oxycodone for cancer-related pain. Cochrane Database Syst Rev 2015.CD003870. [PubMed]
- Lazzari M, Greco MT, Marcassa C, et al. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: a propensity analysis. Drug Des Devel Ther 2015;9:5863-72. [Crossref] [PubMed]
- Schmidt-Hansen M, Bennett MI, Hilgart J. Oxycodone for Cancer Pain in Adult Patients. JAMA 2015;314:1282-3. [Crossref] [PubMed]
- Pergolizzi JV Jr, Seow-Choen F, Wexner SD, et al. Perspectives on Intravenous Oxycodone for Control of Postoperative Pain. Pain Pract 2016;16:924-34. [Crossref] [PubMed]
- Kim NS, Kang KS, Yoo SH, et al. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J Anesthesiol 2015;68:261-6. [Crossref] [PubMed]
- Lassen CL, Link F, Lindenberg N, et al. Anesthesiological acute pain therapy in Germany: telephone-based survey. Anaesthesist 2013;62:355-64. [Crossref] [PubMed]
- Lenz H, Sandvik L, Qvigstad E, et al. A comparison of intravenous oxycodone and intravenous morphine in patient-controlled postoperative analgesia after laparoscopic hysterectomy. Anesth Analg 2009;109:1279-83. [Crossref] [PubMed]
- Koch S, Ahlburg P, Spangsberg N, et al. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand 2008;52:845-50. [Crossref] [PubMed]
- Silvasti M, Rosenberg P, Seppälä T, et al. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand 1998;42:576-80. [Crossref] [PubMed]
- Kalso E, Pöyhiä R, Onnela P, et al. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand 1991;35:642-6. [Crossref] [PubMed]
- Wang N, Wang Y, Pang L, et al. Effect of preemptive analgesia with intravenous oxycodone in the patients undergoing laparoscopic resection of ovarian tumor. Pak J Med Sci 2015;31:300-3. [Crossref] [PubMed]
- Wang J, Pang L, Han W, et al. Effect of preemptive intravenous oxycodone on low-dose bupivacaine spinal anesthesia with intrathecal sufentanil. Saudi Med J 2015;36:437-41. [Crossref] [PubMed]
- Olkkola KT, Hamunen K, Seppälä T, et al. Pharmacokinetics and ventilatory effects of intravenous oxycodone in postoperative children. Br J Clin Pharmacol 1994;38:71-6. [Crossref] [PubMed]
- Kokki H, Laisalmi M, Vanamo K. Interpleural bupivacaine and intravenous oxycodone for pain treatment after thoracotomy in children. J Opioid Manag 2006;2:290-4. [PubMed]
- Pokela ML, Anttila E, Seppälä T, et al. Marked variation in oxycodone pharmacokinetics in infants. Paediatr Anaesth 2005;15:560-5. [Crossref] [PubMed]
- Grant S, Aitchison T, Henderson E, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest 1999;116:1208-17. [Crossref] [PubMed]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. [Crossref] [PubMed]
- Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol 2014;14:75. [Crossref] [PubMed]
- Donders AR, van der Heijden GJ, Stijnen T, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087-91. [Crossref] [PubMed]
Cite this article as: Tu W, Chen Y, Chen B, Peng W, Zhang Y, Lan Z, Du G. Efficacy and safety of intravenous oxycodone for general anesthesia in children. J Emerg Crit Care Med 2017;1:4.