Bloodstream infection caused by Cupriavidus gilardii in a systemic lupus erythematosus patient with invasive pulmonary aspergillosis
Introduction
Systemic lupus erythematosus (SLE) is the most common form of autoimmune disease which is vulnerable to funguses and bacteria (1). Microorganism colonized in respiratory tract and lung is a leading cause of pneumonia when glucocorticoid and immunosuppressor are prescribed (2). Aspergillus isolated in SLE patients from sputum culture is not uncommon and Cupriavidus gilardii is also occasionally isolated from the sputum sample. However, it is difficult to identify Cupriavidus gilardii accurately and the clinical significance of the pathogen is uncertain. In addition, bloodstream infection (BSI) caused by Cupriavidus gilardii due to invasive pulmonary aspergillosis (IPA) has never been reported in the literature.
Case presentation
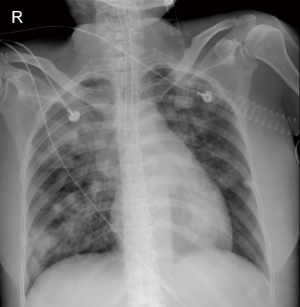
A 52-year-old female was admitted to our intensive care unit (ICU) with cough, hemoptysis and fever for about one month from the rescue room. Her medical history included SLE for three years which was treated with daily oral prednisone 40 mg. On physical examination, she appeared cyanotic with fever up to 39.2 °C and moist rale could be clearly heard on the lower lobe of both sides. Chest X-ray images after intubation showed the cloudiness on both sides (Figure 1). Respiratory failure was managed with protective mechanical ventilation (tidal volume 360 mL, plateau pressure 25 cmH2O and positive end-expiratory pressure 15 cmH2O) (3). Laboratory evaluation at the time of ICU admission revealed negative blood cultures and normal procalcitonin (0.08 ng/mL). However, the level of galactomannan (GM) reached up to 2.54 µg/L.
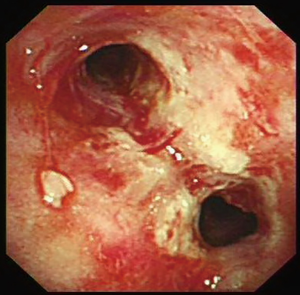
Bronchoscopy was performed through the endotracheal intubation. Hyperemia and bleeding can be detected with pseudomembrane formation around tracheal (Figure 2). Aspergillus fumigatus was isolated from the culture of bronchoalveolar lavage fluid (BALF) sample (Figure 3A,B,C) and the level of GM in BALF sample was 4.33 µg/L which was well above the normal range. Therefore, IPA was probably diagnosed and voriconazole was initiated (intravenous medication, 200 mg q12 h). Another bacteria was also isolated from the BALF sample, which appeared as very small colonies on 5% sheep blood agar after incubation at 24 h in normal atmosphere at 35 °C (Figure 3A). However, the strain could not be identified by Vitek 2 (BioMérieux, Inc) through biochemical testing.
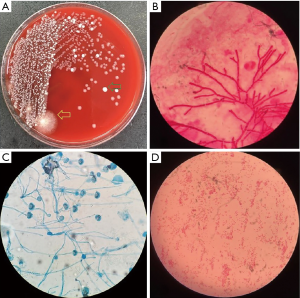
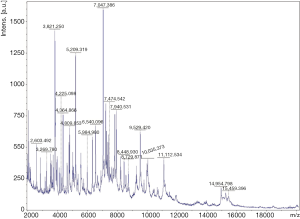
Hemoptysis was still heavily and the patient became febrile to 40.1 °C the next week with chills. The concentration of procalcitonin in serum reached to 46.2 ng/mL and one gram-negative bacteria was isolated from the culture of blood (Figure 3D) which could not be identified by Vitek 2 again. Matrix assisted desorption ionization time of flight mass spectrometry (MALDI-TOF MS, bioMérieux Inc., Durham, NC, USA) was performed and Cupriavidus gilardii was identified (with concordance 97%, Figure 4). Further identification was performed by 16S rRNA gene sequencing which revealed the same result.
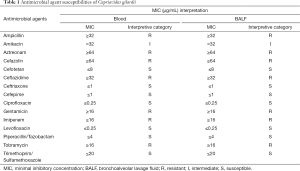
Antimicrobial susceptibility testing was performed and the breakpoints (susceptible, intermediate, or resistant) was determined according to Pseudomonas aeruginosa M100-S27 provided by the Clinical and Laboratory Standards Institute (CLSI) standards. Minimal inhibitory concentrations (MICs) of antibiotics were identical in blood and BALF sample. Ciprofloxacin was prescribed (intravenous medication, 400 mg q8 h) according to the results of antimicrobial susceptibility test (Table 1). However, the culture of blood was still positive three days after antibiotics prescription and the concentration of PCT did not reduce. One week later, she still developed a more serious condition and was maintained on vasopressor support but ultimately succumbed to multiorgan failure and died after two weeks.
Full table
Discussion
Cupriavidus gilardii is a nonfermenting gram-negative bacillus which can be isolated from contaminated soils or plants (4). This sort of bacteria can also be identified as a rare colonizer in upper respiratory tract and seldom cause infection. Furthermore, clinical materials about Cupriavidus gilardii were not well documented due to difficulty in accurately identifying the species. However, this organism may represent an emerging pathogen in immunocompromised patients due to its innate antimicrobial resistance and its ability to acquire new resistances as it colonizes in human host (5). Therefore, more and more attention should be paid to this bacteria, especially in patients with immunosuppression.
IPA is often develops in severely immunocompromised patients when glucocorticoid and immunosuppressor are prescribed in those with asymptomatic aspergillosis colonization (6). Lung tissue and capillary were impaired by hyphae during the progress of IPA which could demonstrate on histology. Pulmonary vascular invasion is involved in the pathogenesis in hosts and is responsible for the higher frequency of dissemination to other organs such as eyes, liver and skin (7). Sometimes, colonization bacteria may circulated through the bloodstream by the damaged vessels and cause BSI like our patient.
Timely, appropriate treatment of infection depends on rapid, specific identification of causative microorganisms. Traditionally, microbial identification involves morphologic characterization by microscopy and staining, growth in culture, phenotypic and metabolic characterization by biochemical tests. Up to now, it was reported that difficulty still remained in correctly classifying Cupriavidus gilardii by biochemical (5). Gene sequencing like nanopore, 16S rRNA and next generation sequencing (NGS) are methods for identifying rare bacteria species with high precision up to now. However, complicated operation and high cost restrict their usage especially in the species-level discrimination. The technology of MALDI-TOF MS develops rapidly in the last decade which leads to the routine use in a part of clinical microbiology laboratory. Rare pathogens are identified via ribosomal proteins compare. Therefore, a considerable number of organism is needed in the process of MALDI-TOF MS (8,9).
A review of literature for characteristics in cases of infection caused by Cupriavidus gilardii is summarized as below (Table 2). Only three cases (10-12) were reported.

Full table
In summary, pulmonary vascular invasion is involved in the pathogenesis in hosts of IPA which can induce BSI by some rare pathogens like Cupriavidus gilardii. Identification of this strain is difficult using typical methods such as biochemical testing which may delay the initiation of treatment and lead to poor prognosis. MALDI-TOF MS might be a better choice in rare bacteria identification which is accurate, rapid with cost-saving.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the family members of the patient for publication of this manuscript.
References
- Chen D, Xie J, Chen H, et al. Infection in Southern Chinese Patients with Systemic Lupus Erythematosus: Spectrum, Drug Resistance, Outcomes, and Risk Factors. J Rheumatol 2016;43:1650-6. [Crossref] [PubMed]
- Sciascia S, Cuadrado MJ, Karim MY. Management of infection in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2013;27:377-89. [Crossref] [PubMed]
- Fuller BM, Mohr NM, Carpenter CR. Protective ventilation for patients without acute respiratory distress syndrome. JAMA 2013;309:654. [Crossref] [PubMed]
- Vandamme P, Coenye T. Taxonomy of the genus Cupriavidus: a tale of lost and found. Int J Syst Evol Microbiol 2004;54:2285-9. [Crossref] [PubMed]
- Coenye T, Goris J, De Vos P, et al. Classification of Ralstonia pickettii-like isolates from the environment and clinical samples as Ralstonia insidiosa sp. nov. Int J Syst Evol Microbiol 2003;53:1075-80. [Crossref] [PubMed]
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015;70:270-7. [Crossref] [PubMed]
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-e60. [Crossref] [PubMed]
- Turhan O, Ozhak-Baysan B, Zaragoza O, et al. Evaluation of MALDI-TOF-MS for the Identification of Yeast Isolates Causing Bloodstream Infection. Clin Lab 2017;63:699-703. [Crossref] [PubMed]
- Warnke P, Köller T, Stoll P, et al. Nosocomial infection due to Enterococcus cecorum identified by MALDI-TOF MS and Vitek 2 from a blood culture of a septic patient. Eur J Microbiol Immunol (Bp) 2015;5:177-9. [Crossref] [PubMed]
- Karafin M, Romagnoli M, Fink DL, et al. Fatal infection caused by Cupriavidus gilardii in a child with aplastic anemia. J Clin Microbiol 2010;48:1005-7. [Crossref] [PubMed]
- Kobayashi T, Nakamura I, Fujita H, et al. First case report of infection due to Cupriavidus gilardii in a patient without immunodeficiency: a case report. BMC Infect Dis 2016;16:493. [Crossref] [PubMed]
- Tena D, Losa C, Medina MJ, et al. Muscular abscess caused by Cupriavidus gilardii in a renal transplant recipient. Diagn Microbiol Infect Dis 2014;79:108-10. [Crossref] [PubMed]
Cite this article as: Zhu C, Guo R, Zhou M, Yu Y. Bloodstream infection caused by Cupriavidus gilardii in a systemic lupus erythematosus patient with invasive pulmonary aspergillosis. J Emerg Crit Care Med 2018;2:46.